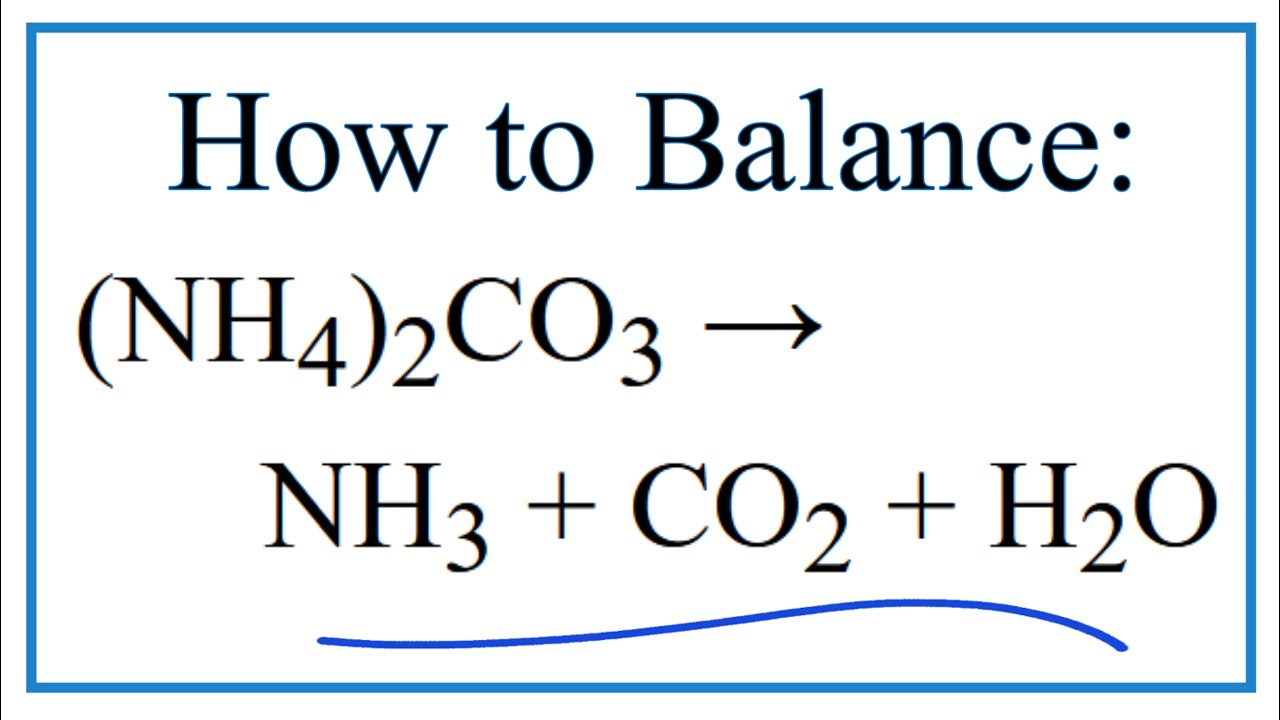
Best Ammonium Carbonate Balanced Equation

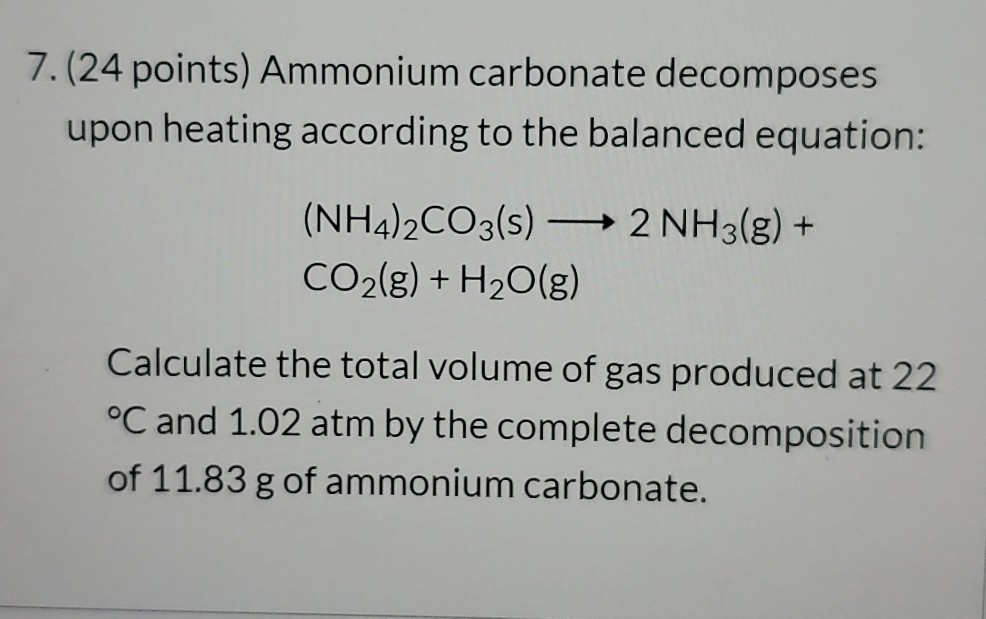
Problem Details Ammonium carbonate decomposes upon heating according to the following balanced equation.
Ammonium carbonate balanced equation. Question 22 of 40 Complete the balanced molecular chemical equation for the decomposition reaction that takes place as solid ammonium carbonate when heated. Immediate steps should be taken to limit spread to the environment. Answer to Question 22 of 40 Complete the balanced molecular.
IronII sulfate ammonium sulfide Fe SO 4 NH 4 2 S Fe S NH 4 2 SO 4. What is the balanced equation for ammonium carbonate and nickel II chloride. NH42CO3 Then write an unbalanced equation.
Potassium carbonate barium chloride potassium chloride barium carbonate K2CO3 BaCl2 2KCl BaCO3 magnesium hydroxide sulfuric acid magnesium sulfate water MgOH2 H2SO4 MgSO4 2HOH. A Solid ammonium carbonate decomposes as it is heated. You may use the empty space at the bottom of the next page for scratch work but only equations that are written in the answer boxes provided will be graded.
The products of the combustion of acetylene are carbon dioxide and water vapor. CaSO 4 NH 4 2 CO 3 CaCO 3 NH 4 2 SO 4 Check the balance Calcium sulfate react with ammonium carbonate to produce calcium carbonate and ammonium sulfate. Which choice of reactant quantities shown below would result in the greatest amount of.
Write the balanced COMPLETE ionic equation for the reaction when ammonium carbonate and calcium chloride are mixed in aqueous solution. Ammonium carbonate appears as a colorless crystalline solid or a white powder with a strong odor of ammonia. Fe Au Co Br C O N F.
Ionic charges are not yet supported and will be ignored. If no reaction occurs simply write only NR. Acetylene gas C2H2 is often used by plumbers welders and glass-blowers because it burns in oxygen O2 with an intensely hot white flame.