Great Balanced Equation For Decomposition Reaction

CISCE ICSE Class 9.
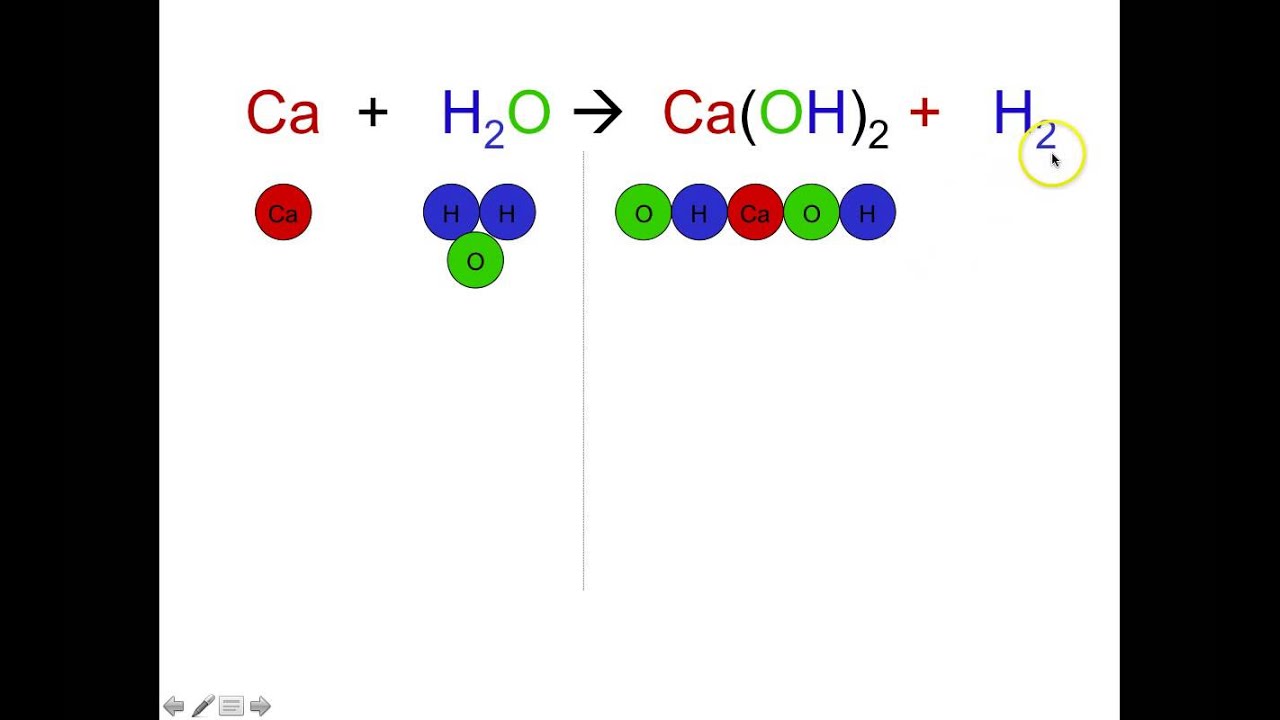
Balanced equation for decomposition reaction. Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen andthe breakdown of water to hydrogen and oxygen. The following diagram represents the zeroth-order decomposition of A to form X according to the following balanced chemical equation. Balance the chemical equation for the decomposition reaction that occurred in Experiment 2 by specifying the coefficients that should replace the letters.
ACu2Co3 OH2 s bH2O s heat cCuO s dH2O l eCO2 g. Limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Fe Au Co Br C O N F.
Examples of decomposition reactions include the breakdown of hydrogen peroxide to water and oxygen and. You balance decomposition reactions just like any other reaction. Enter either the number of moles or weight for one of the compounds to compute the rest.
This can be represented by the general equation. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. 2 NaHCO3 s Na2CO3 s CO2 g H2O g Like most chemical reactions the rate of the reaction depends on temperature.
Advertisement Remove all ads. Summary A decomposition reaction occurs when one reactant breaks down into two or more products. AB A B.
Oscillations Redox Reactions Limits and Derivatives Motion in a Plane Mechanical Properties of Fluids. Two new compounds are generated from single reactant in chemistry through a chemical reaction which. A thermal decomposition reaction in which a metallic.