Great Double Displacement Reaction Wikipedia

Usually a double displacement reaction results in precipitate formation.
Double displacement reaction wikipedia. The chemical bonds between the reactants may be. Classically chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms with no change to the nuclei no change to the elements present and can often be described by a chemical equation.
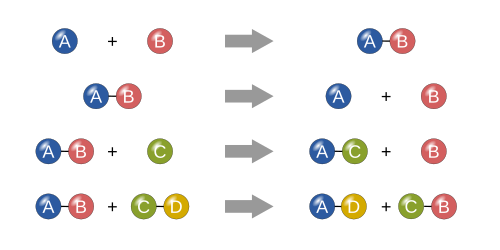
A displacement reaction is a type of reaction in which part of one reactant is replaced by another reactant. Double displacement reactions generally take place in aqueous solutions in which the ions precipitate and there is an exchange of ions. Neutralisation is a rather limited reaction.
Usually a double displacement reaction results in precipitate formation. For example on mixing a solution of barium chloride with sodium sulphate a white precipitate of 27 Feb 2015 Transcript of Double Displacement Lab. Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions.
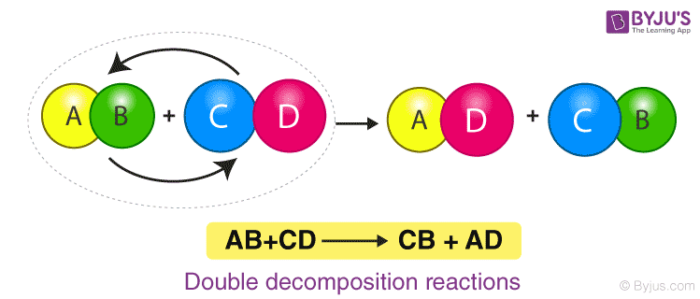
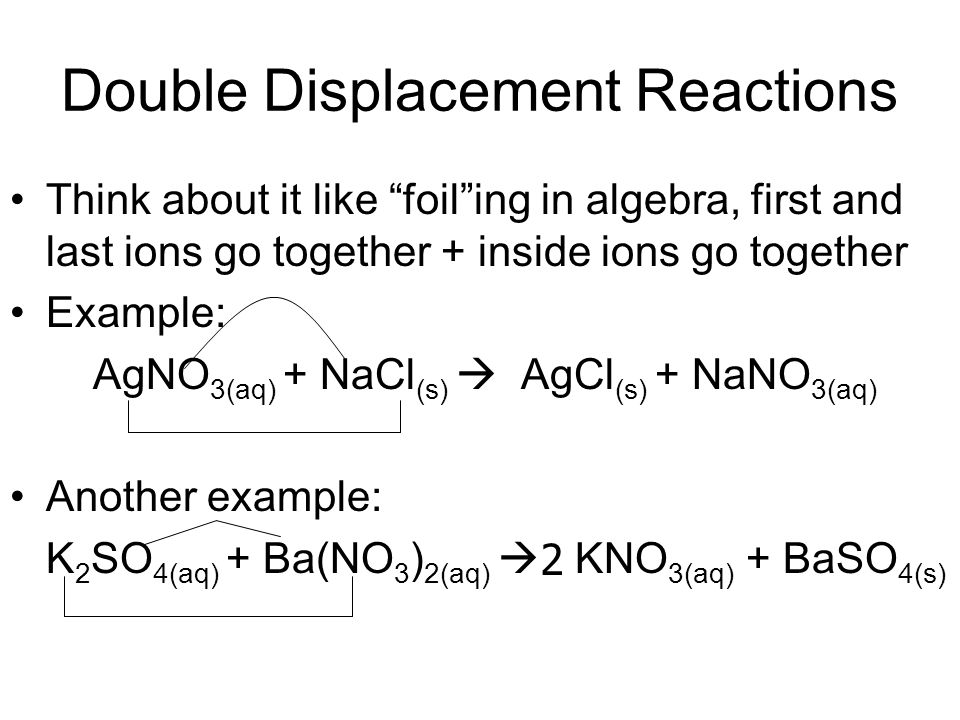
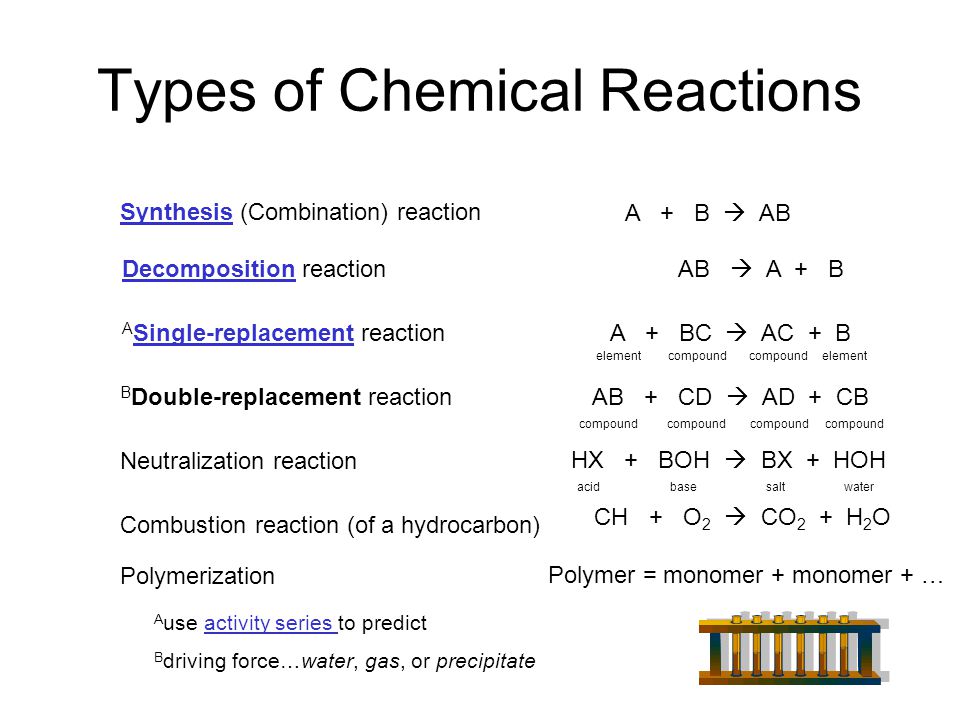
A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products. Double displacement reactions are those reactions where the cations and anions of reactants switch places with each other or replaces each other. A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
A displacement reaction is also known as a replacement reaction or a metathesis reaction. There are two types of displacement reactions. RelistedJenks24 0704 22 June 2012 UTC Whoop whoop pull up Bitching Betty Averted crashes 1834 14 June 2012 UTC.
Generally it can be represented as follows AB CD. Please do not modify it.



/experiment-showing-how-miscible-liquids-react-the-coloured-pigments-diffuses-over-time-until-evenly-distributed-in-the-water-creating-a-mixture-of-the-two-colours-123535153-572f8ecc5f9b58c34cabc582.jpg)