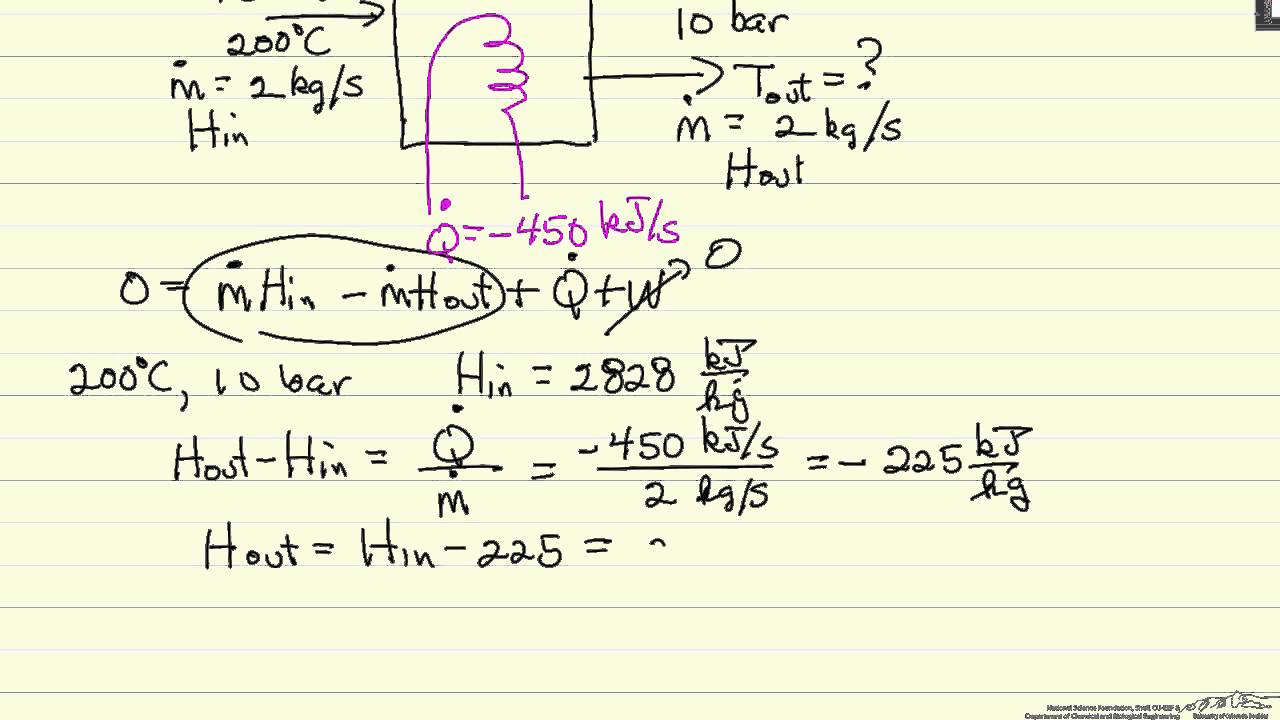Looking Good Energy Balance Equation Chemical Engineering

Once mass and energy balance.
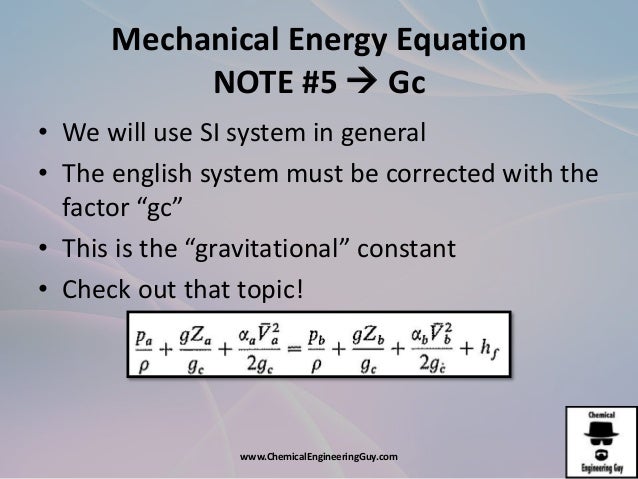
Energy balance equation chemical engineering. Recall that our mechanical energy balance is P ρ 1 2 α v a v g 2 g z 2 P ρ 1 2 α v a v g 2 g z 1 w s w f When applying this equation it is important to remember that we must choose locations such that point 1 is upstream of point 2. These two laws of physics provide the basis for two tools which are used routinely in environmental engineering and science---the mass balance and the energy balance. Mass balances are developed and applied in some detail in the following section after which the concept of the energy balance.
Energy balance For any system the energy going into the system must equal the energy coming out of the system plus any accumulation of energy in the system Only ONE energy balance equation is written for any system or sub-system irrespective of the number of components in the products Note. The general balance equation states that the total or component mass or energy of any system can be modeled by. 4GATE 2018 Chemical Engineering - Centrifugal Pump1.
Mechanical Energy Balance Special Case No temperature change U 0 No chemical reaction H 0 Velocity pressure and friction are important Energy equation reduces to the mechanical energy equation 6 æ Pressure change Velocity change Height change Friction Shaft work. Two methods are shown for computing a change in enthalpy when there are reactions. Introduction to Degrees of Freedom mirror.
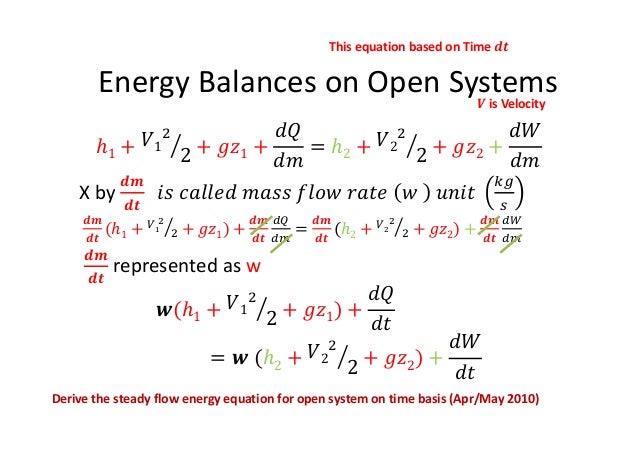
Material Balance Problem Approach mirror. Energy Balances As with mass balances it is useful to start with our initial definition of a balance equation. Our generalized conservation of energy equation is therefore output streamsmˆU 1 2αv2 gzout input streamsmˆU 1 2αv2 gzin Q W Note that the internal energy is often combined with the flow work into a property called the enthalpy which is represented by the symbol ˆH and defined as ˆH.
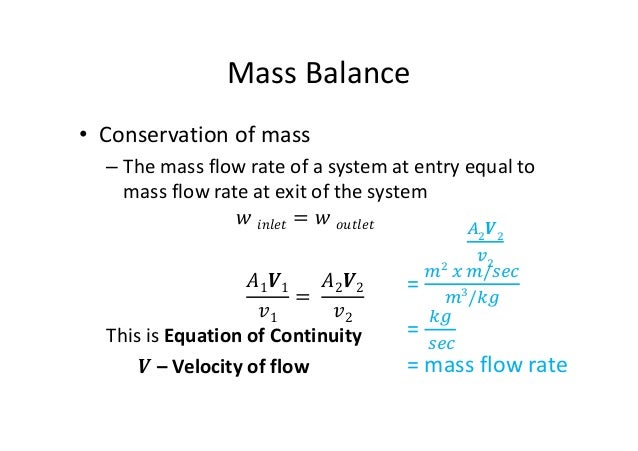
Liquid-Liquid Extraction Material Balance mirror. This portion of the course deals with these tools. For the A.
In this course LearnChemE covers engineering calculations process variables single unit material balances multiple-unit material balances equations of state phase equilibrium energy balances and balances involving reactions. 2B stoichiometry we substitute the rate expression and 1 into Equation 617 to obtain C V dT dt H R RT kn A in which C V V R CV is the total constant-volume heat capacity. Q W U E E U E E f kf pf i ki pi energy transferred final system energy initial system energy Accumulation.