Peerless How To Calculate Percentage Uncertainty In Chemistry Ib

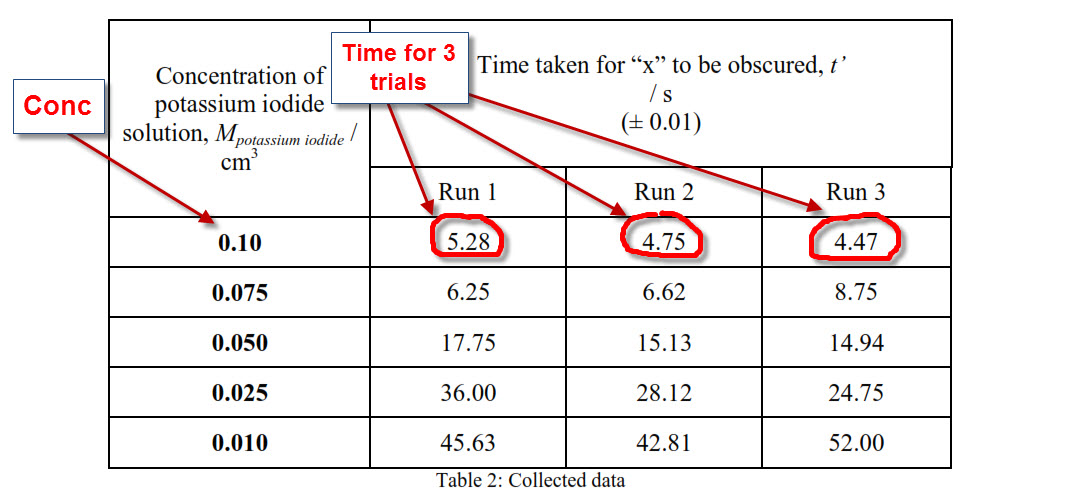
Uncertainty Calculation 2 fold Serial dilution using 3 H 2 O 2 Two Methods used 1 st Uncertainty Method For 15.
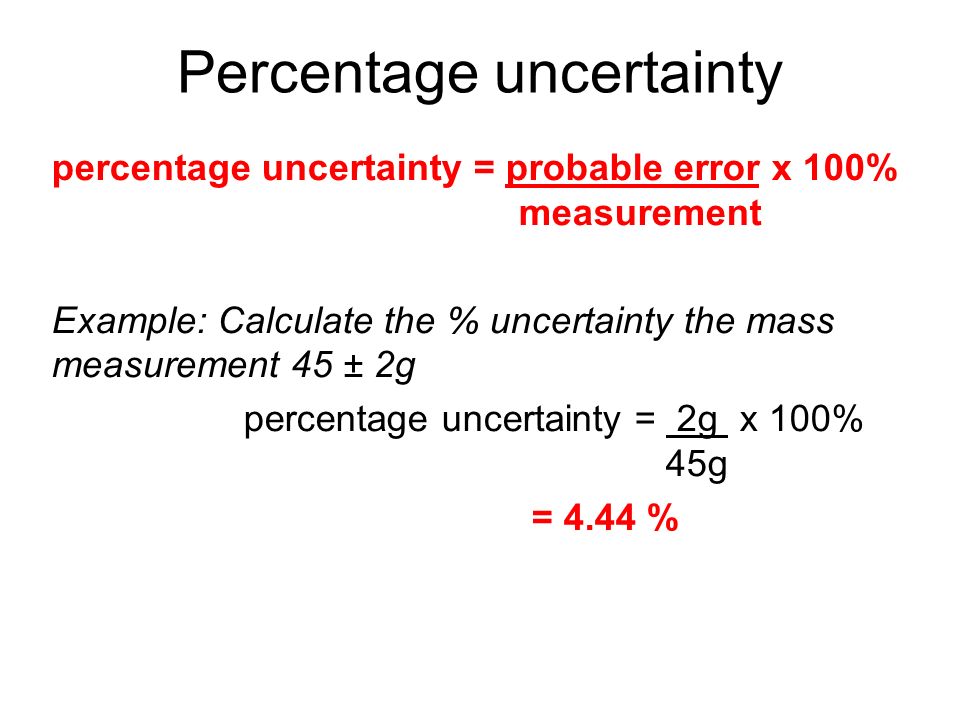
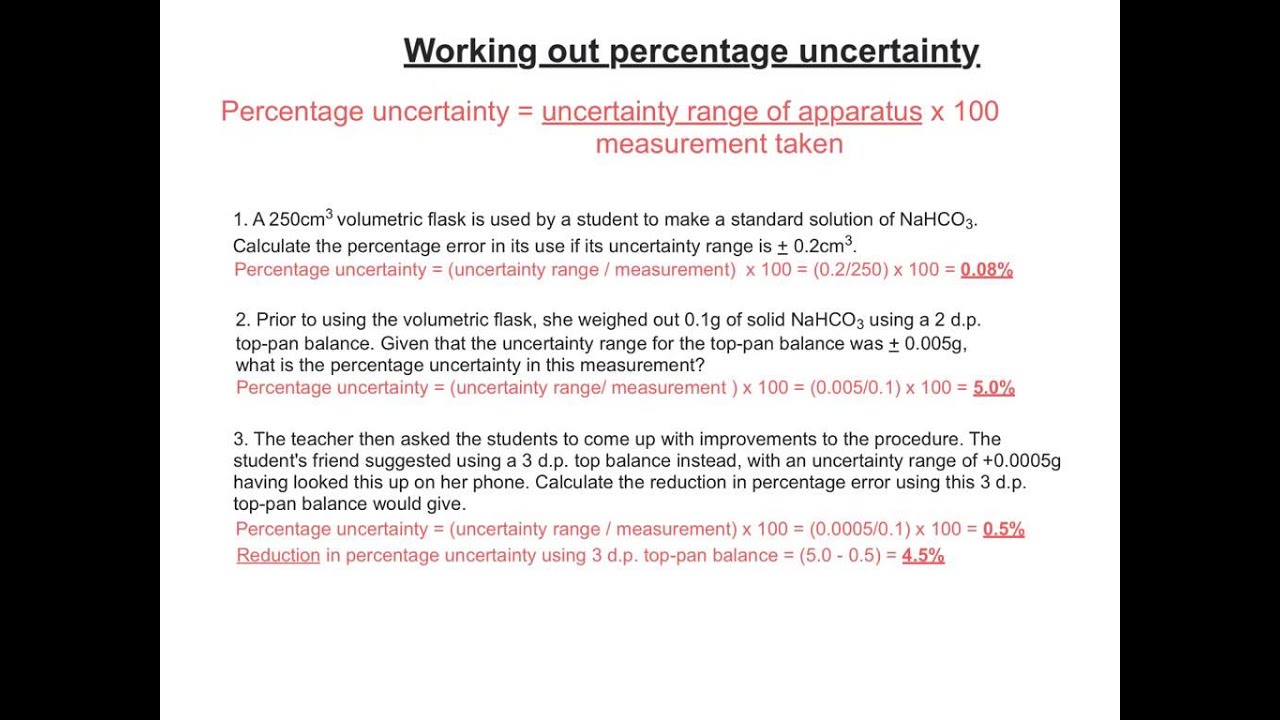
How to calculate percentage uncertainty in chemistry ib. How to calculate uncertainty in chemistry ib Home. The propagated uncertainty for the total volume is 005 cm3 005 cm3 01 cm3. You just clipped your first slide.
This is then multiplied by one hundred. Total Uncertainty is 16 Answer 15 16 Propagation of error involved 11. This digital version of Chemistry for the IB Diploma Coursebook Second edition comprehensively covers all the knowledge and skills students need during the Chemistry IB Diploma course for first examination in 2016 in a reflowable format adapting to any screen size or device.
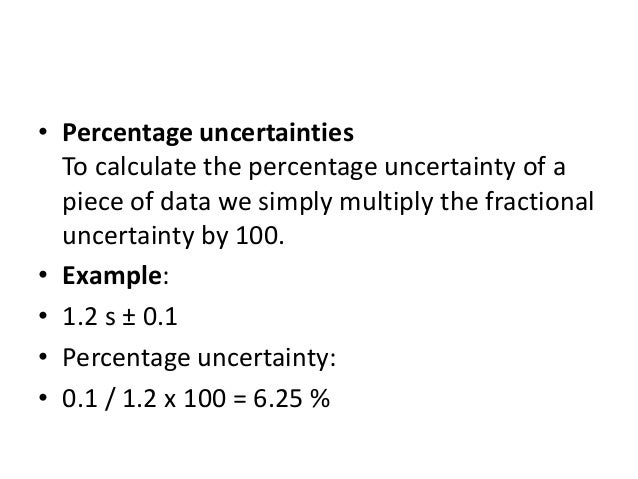
Clipping is a handy way to collect important slides you want to go back to later. Percentage uncertainty A percentage by definition is a value out of a potential hundred. Accuracy is how close your experimental value is to your to the literature value which is measured by error.
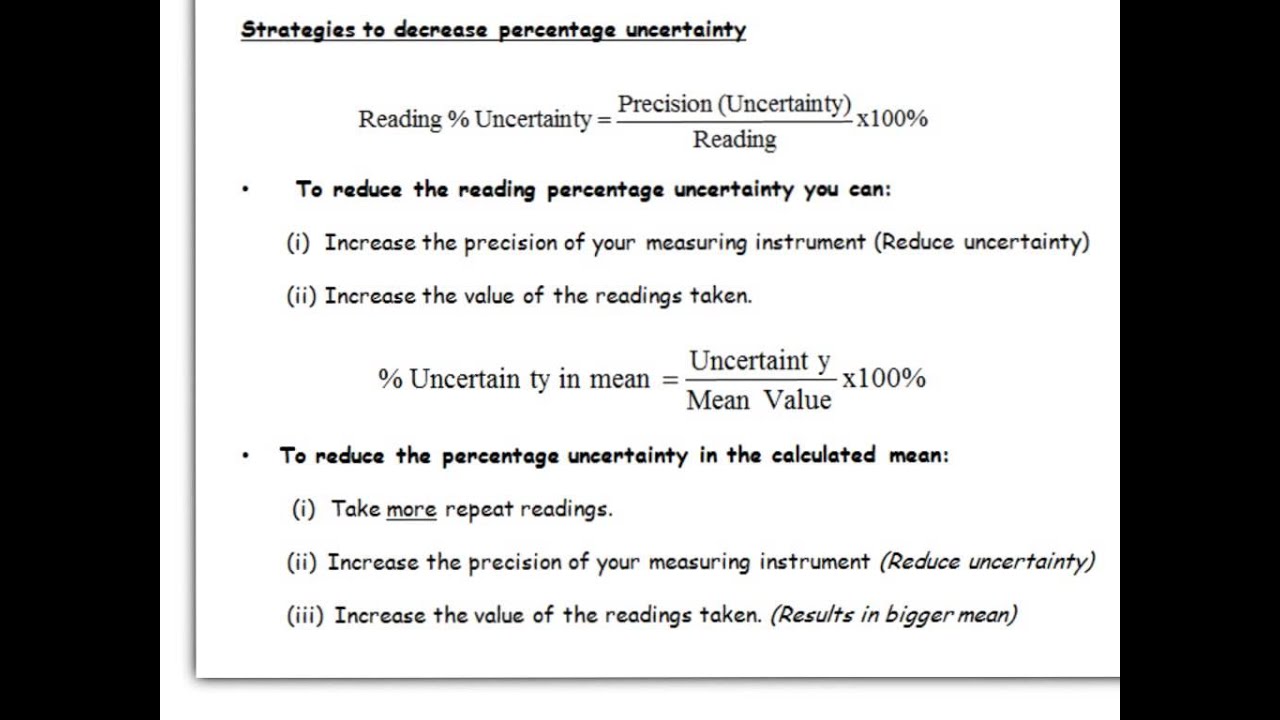
Multiplication or division of data means that the percentage uncertainties must be ad. Calculate the difference of each trial with the average. Uncertainty is the equal chance of measuring something too high or too low which is measured by uncertainty unc.
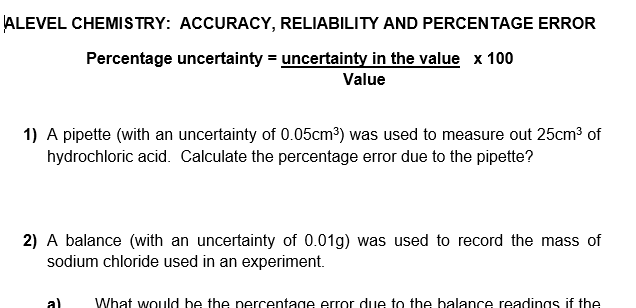
Assuming the uncertainty due to the burette and the volumetric flask is 050 and 010 respectively the overall uncertainty is obtained by summing all the individual uncertainties. Here are the questions. The percentage is calculated by taking the absolute error in a measurement and dividing by the value of the measurement itself.
The only two methods left are 1 and 2. IB ChemistryUncertainty Error Analysis Standard Deviation Uncertainty Calculation for Rate and Concentration of reaction. In most situations I recommend you to choose the method that gives the highest uncertainty.