Fun K2o And Water Reaction

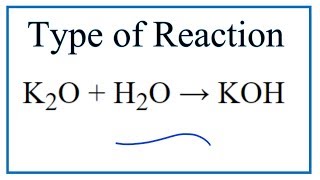
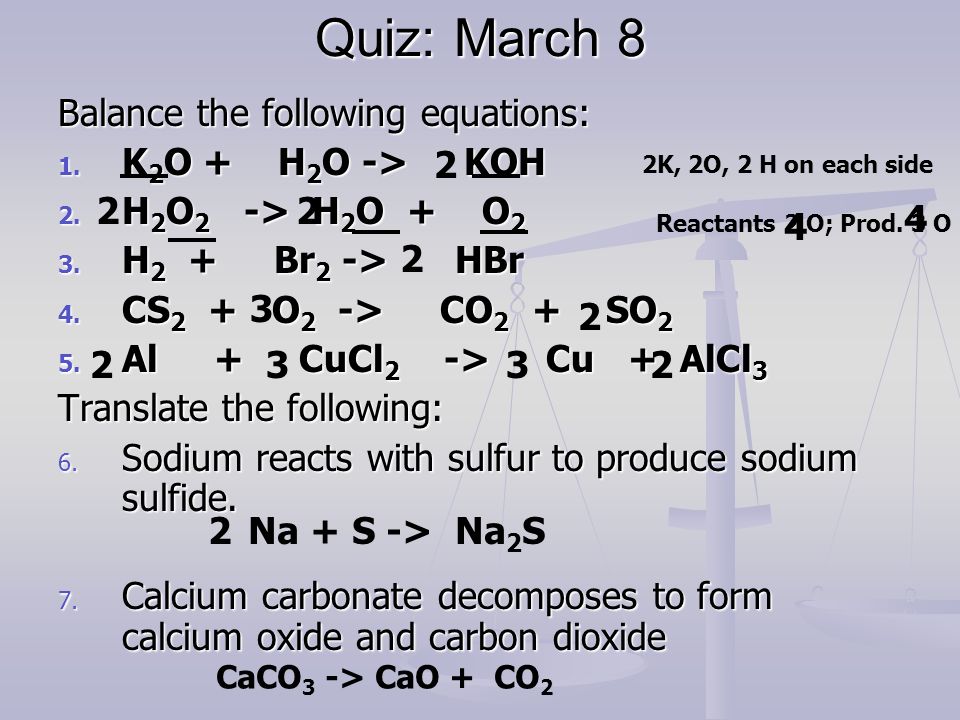
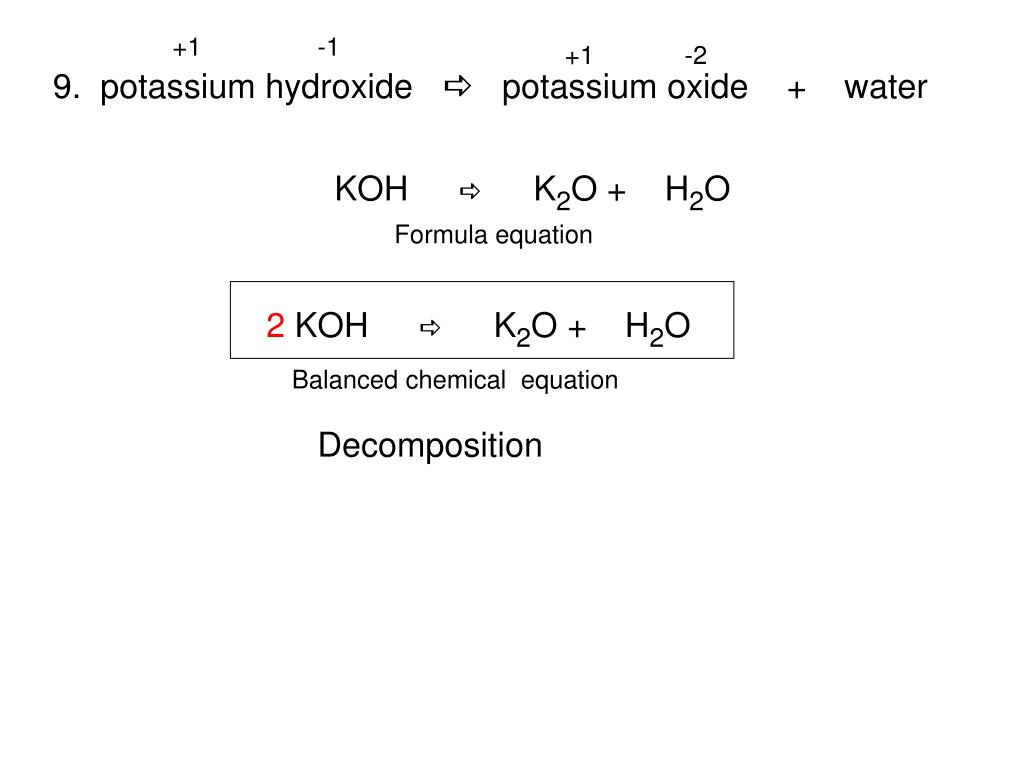
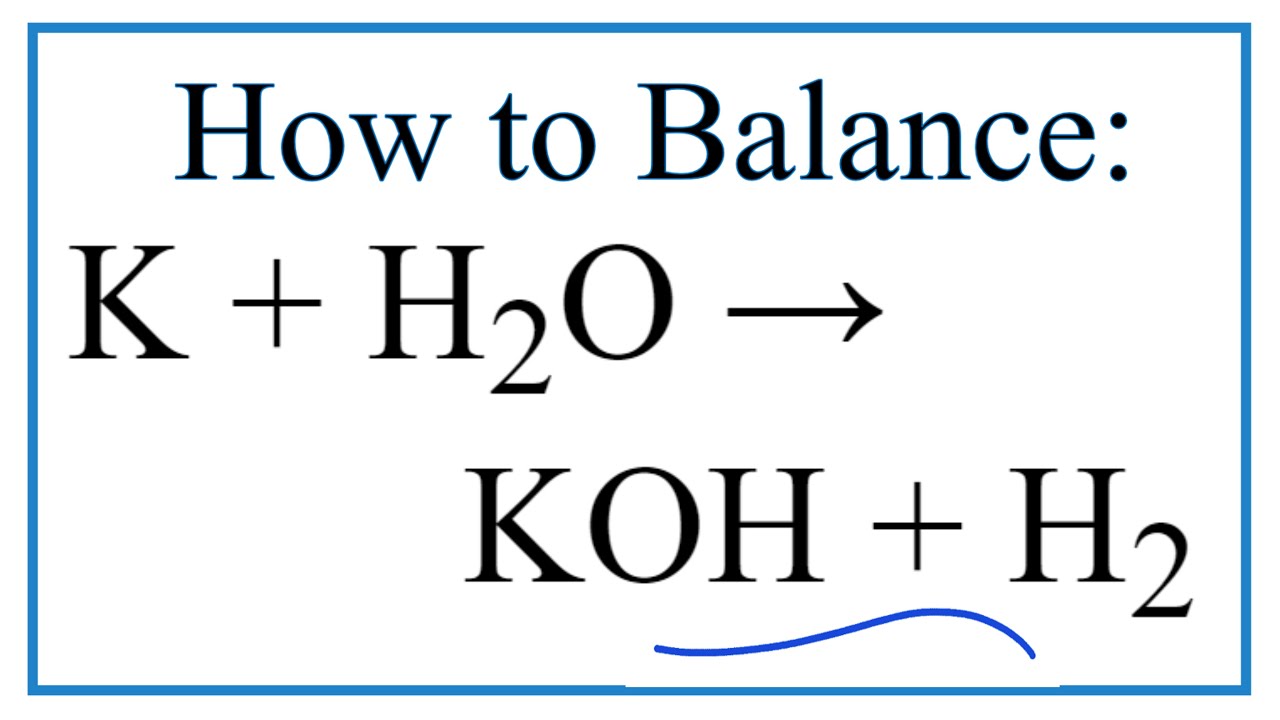
K2O s H2O l 2KOH aq Potassium oxide solid plus water potassium hydroxide.
K2o and water reaction. A Write the molecular and netionic equations for this reaction. Na 2 O 2HCl -- 2NaCl H 2 O easiest way to do this is to balance this equation is to write down all the atoms on both sides of the reaction. Since oxygen is naturally diatomic the total number of atoms of each element is now the same on both sides of the equation so it is balanced.
K2O is a basic oxide and reacts with water violently to produce the caustic potassium hydroxide. Potassium oxide water produces potassium hydroxide. We need 2 potassium ions to balance one oxide ion making the formula K2O.
As K20 dissolves in water the oxide ion reacts with water molecules to form hydroxide ions. Fluorine and Water Reaction F2 H2O. First we balance the molecular equation.
Potassium oxide is an ionic compound. Fluorine F 2 the most electronegative element reacts with water in a different way compared to other halogens doAccording to the amount of fed and rate of feeding to water products given by the reaction may vary. The potassium has a charge of K and oxygen has a charge of O2.
This reaction is of the spontaneous decomposition of hydrogen peroxide down into water and oxygen. It is deliquescent and will absorb water from the atmosphere initiating this vigorous reaction. Write the molecular and net ionic equations for this reaction.
It is deliquescent and will absorb water from the atmosphere initiating this vigorous reaction. Refer to the scenario and balanced equation. This amazingly fresh crisp tasting water comes direct from the blue ridge mountains shipped to your front door.