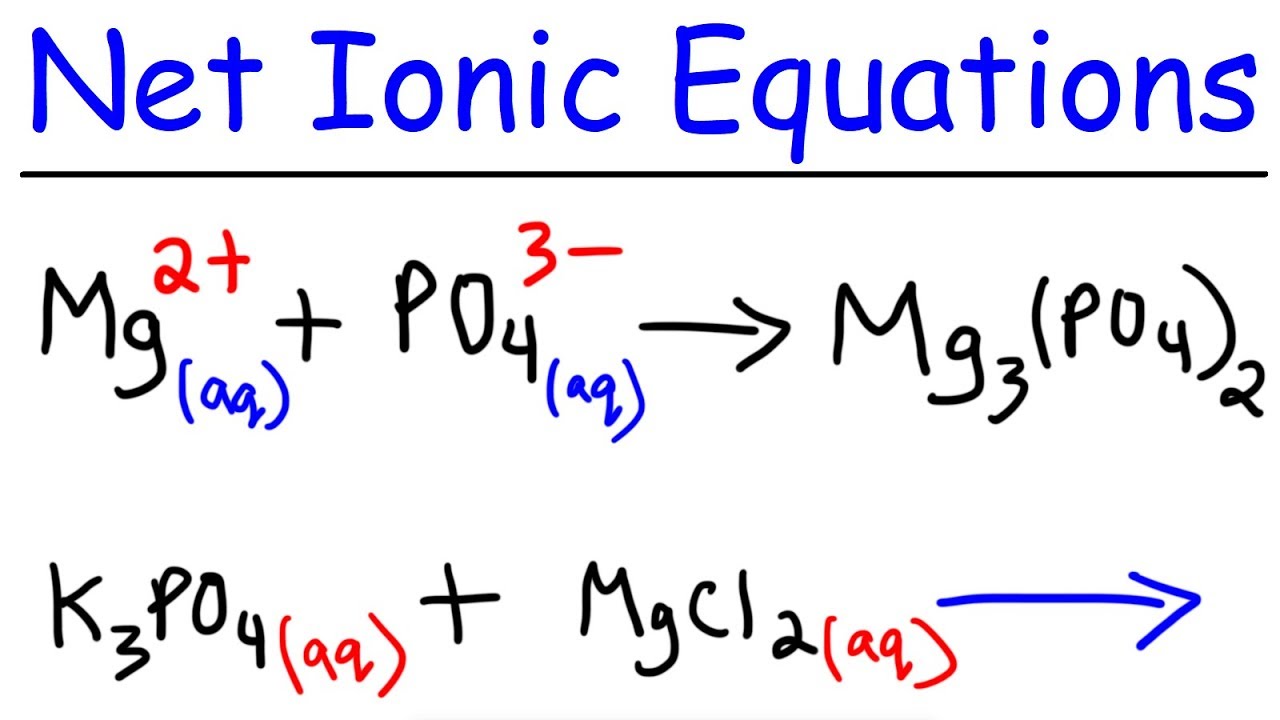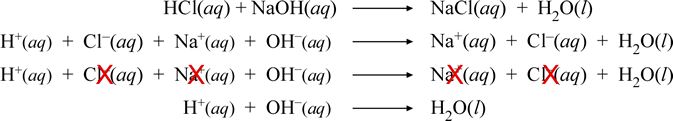Divine Total And Net Ionic Equation Calculator

Net ionic and ionic equation calculator is a free online tool that shows the structure equilibrium constant balanced equation substance properties with chemical formulas and names.
Total and net ionic equation calculator. Writing balanced net ionic equations calculator tessshlo equation lessons examples and solutions how to write a 10 steps with pictures worksheet answers you total easy cute766 solved show why th chegg com. Balancing the molecular equation transforming to a complete ionic equation. Get the free NET IONIC EQUATION CALCULATOR widget for your website blog Wordpress Blogger or iGoogle.
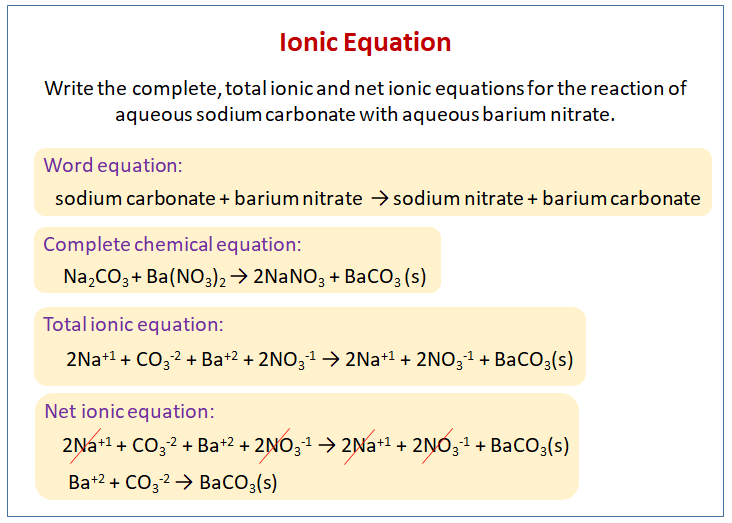
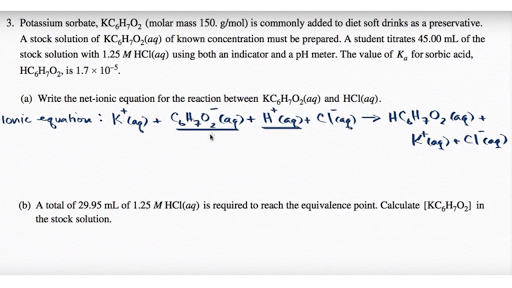
The net ionic equation is the chemical equation that shows only those elements compounds and ions that are directly involved in the chemical reaction. Net Ionic Equation Calculator To write a net ionic equation you have to write the balanced molecular equation. Cross out the present spectator ions.
2 HBraq NH 4 2 CO 3 aq ----- 2 NH 4 Braq H 2 CO 3aq strong soluble soluble unstable acid salt salt substance b Total ionic equation. Potassium carbonate 2K CO 3 2- K 2 CO 3. Enter the ionic chemical compound in the respective input fields.
The balanced net ionic equation calculator tool makes the prediction quick and easier and displays the answer in a fraction of seconds. There are three basic steps to writing a net ionic equation. Finally the ionic formula and the net ionic charge will be displayed in the new window.
They are most commonly used in redox reactions double replacement reactions and acid-base neutralisations. Net ionic equations are an important aspect of chemistry as they represent only the entities that change in a chemical reaction. It is surprising that we can predict the product of the following reaction only by seeing the LHS of the equation.
An ionic equation is a chemical equation in which electrolytes are written as dissociated ions. Notice that in writing the net ionic equation the positively-charged silver cation was written first on the reactant. This way students can see that the ions combine in whole number ratios in order to produce a neutral chemical species.