Spectacular What Is Skeletal Chemical Equation

Writing proper formulas for the species in chemical reactions equations.
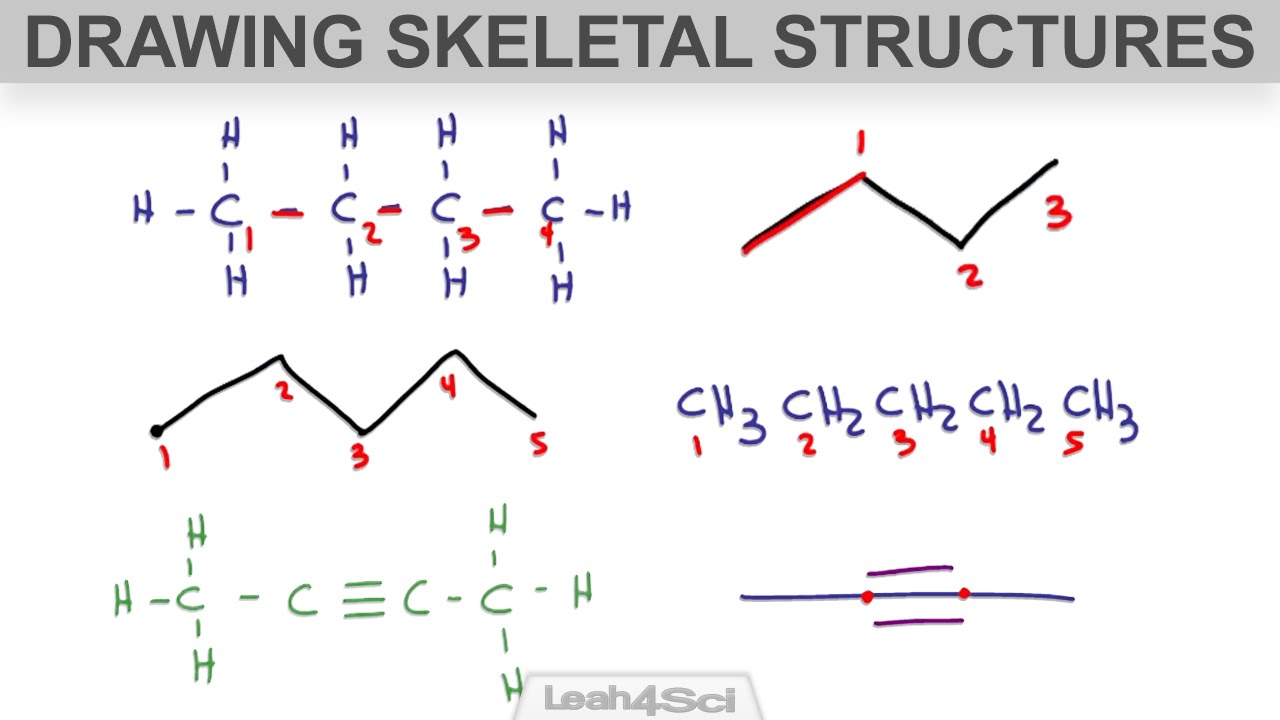
What is skeletal chemical equation. In essence it is identical to a word equation except that the names of the reactants and the products are substituted by their chemical symbols. What is the main element that sets Western art criticism. In a skeleton equation you put chemical formulas in place of chemical names.
An unbalanced equation is a chemical equation in which the total number of atoms of each element on the reactant side is not equal to the number of atoms of the same element on the product side. The correct term used is skeletal chemical equation not skeletal gap equation. Skeletal equation are the equation which represents the reactants and the products involved but which is not balanced in terms of stoichiometric coefficient.
In a skeleton equation you put chemical formulas in place of chemical names. Mg HCl MgCl 2 H 2. A chemical equation in which the number of atoms of reactants are equal to the number of atoms of products is called a balanced equation.
The number of atoms of each element is same. The position of the atoms stays the same. This equation is also called a skeletal equation.
A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. According to the law of conservation of mass mass or atoms are neither created nor destroyed in chemical reactions. Skeletal chemical equation is a representation of a chemical reaction using chemical formulae of reactants and products and it is unbalanced.
This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide. Some examples of this are shown in Figure 1. A chemical equation is said to be skeleton when no attempt are made to balance the equation.