Amazing What Is The Difference Between A Skeleton Equation And A Balanced Chemical Equation
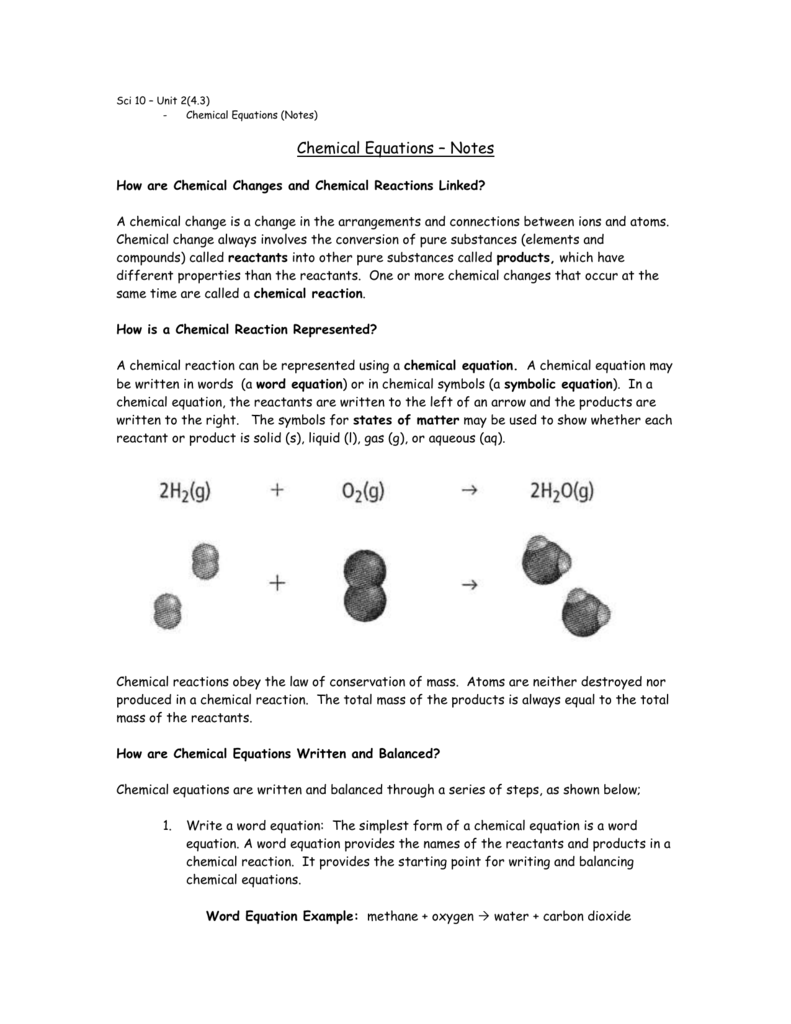
In a word equation the names of the reactants are separated from the names of the products by a yield arrow.
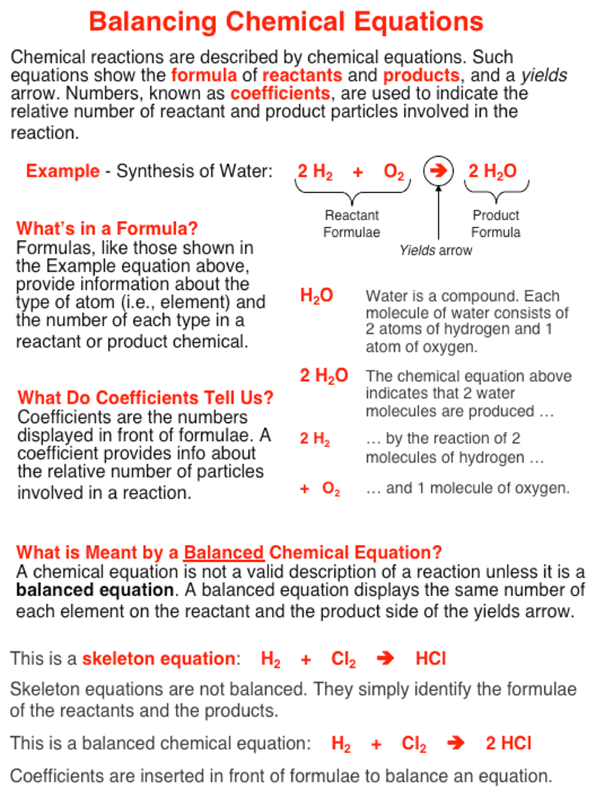
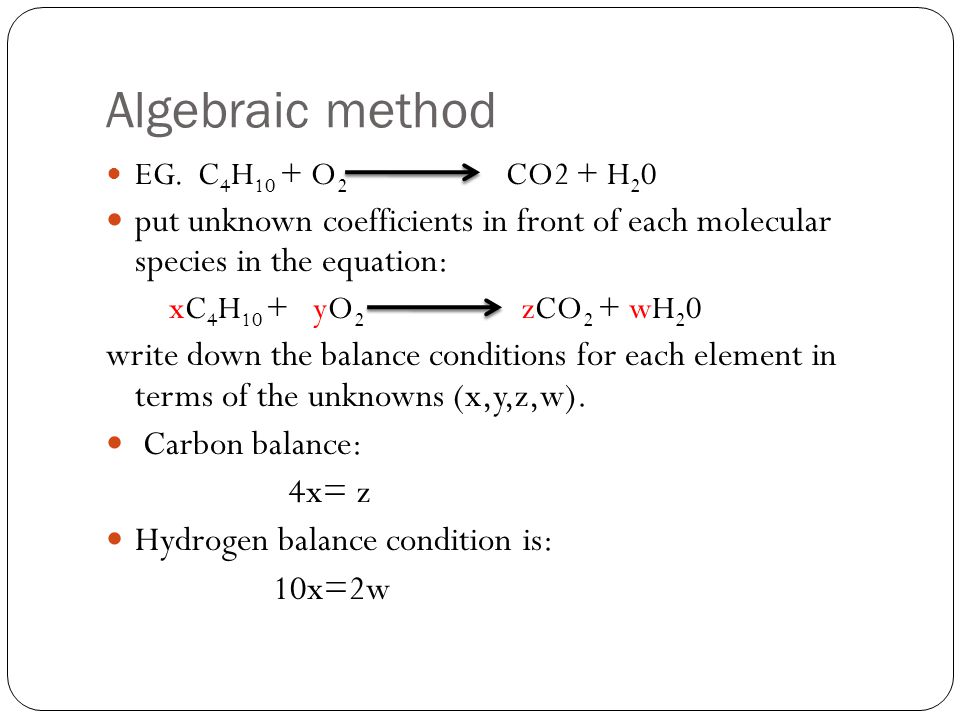
What is the difference between a skeleton equation and a balanced chemical equation. A Skeletal chemical equation should be balanced to obey the law of conservation of mass. The above equation is just a skeletal equation where the formulas are written but the no. Fe Au Co Br C O N F.
Skeletal chemical equation is a representation of a chemical reaction using chemical formulae of reactants and products. A skeletal or skeleton chemical equation is an unbalanced equation. A skeleton equation just shows the reaction a balanced equation shows how many molecules of each reactant are needed and how many molecules of the products are formed Was this answer helpful.
It describes a chemical reaction using the chemical formulas of the reactants and products. A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. In a skeleton equation you put chemical formulas in place of chemical names.
Differentiate between a skeletal chemical equation and a balanced chemical equation. What is the difference between a skeleton equation and a chemical equation. The equation thus obtained is known as a balanced equation.
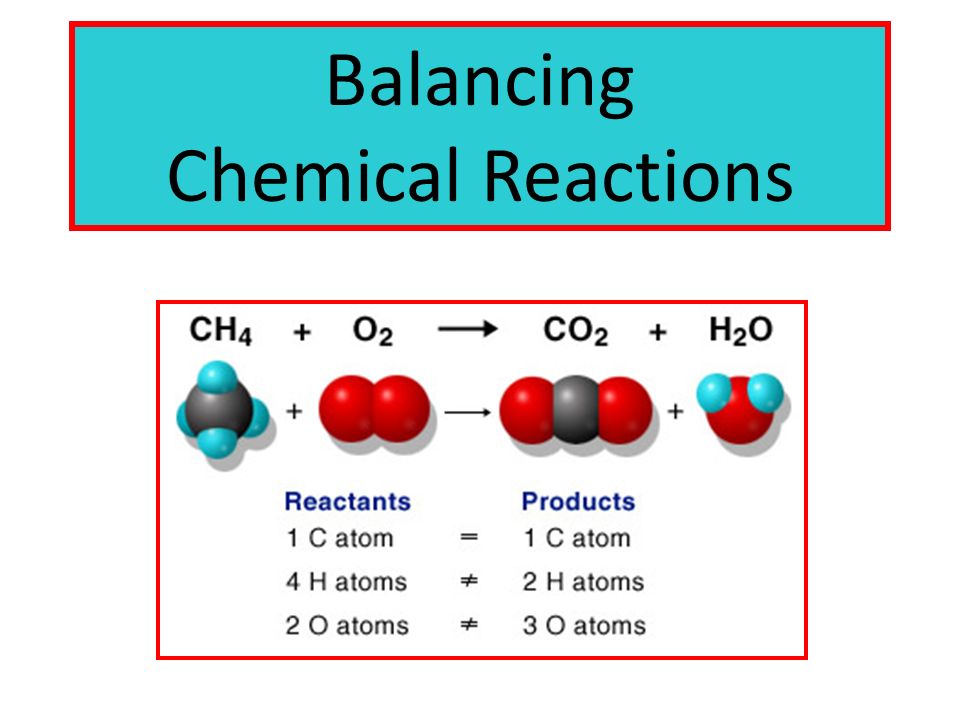
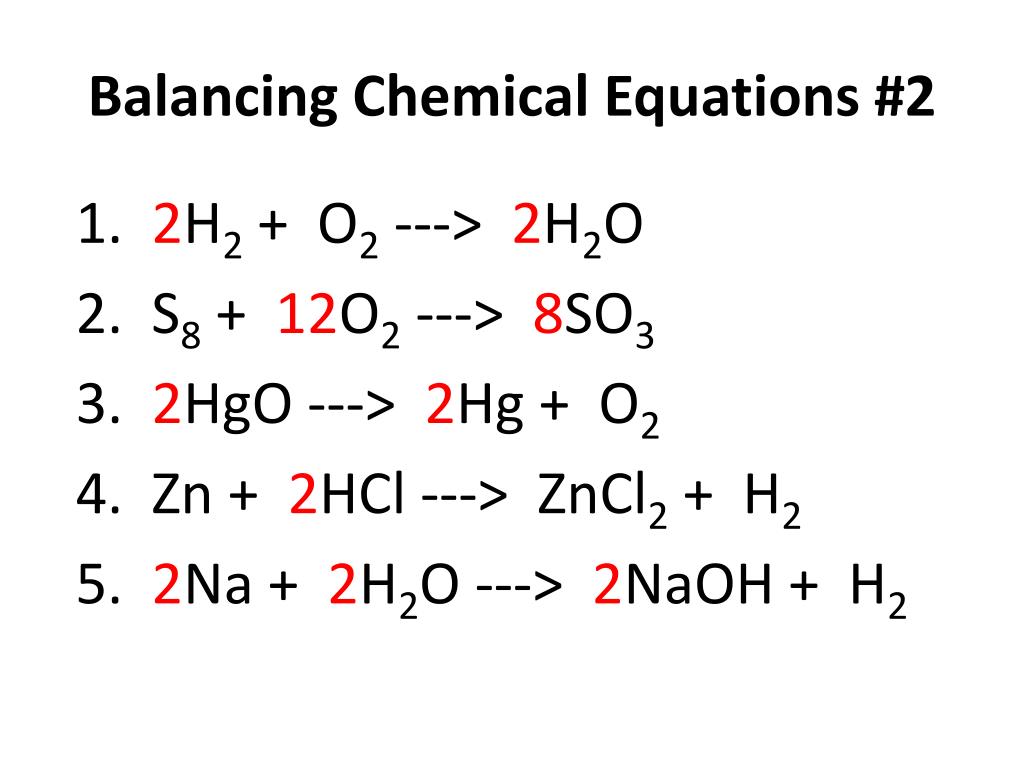
For instance the reaction between sodium and water to form sodium hydroxide and hydrogen can be represented as given below- Na sodium H 2 O water NaOH sodium hydroxide H 2 hydrogen Skeleton Equation. The balanced equation will appear above. In a balanced chemical reaction the number of atoms in each element of a reactant side should be equal to the number of atoms of that element on the product side.
This skeleton equation shows that magnesium reacts with oxygen to form magnesium oxide. A skeleton equation is just a way of using the formulas to indicate the chemicals that were involved in the chemical reaction. Later it has to be balanced by appropriate number of molecules.