Simple Ammonia And Hydrogen Chloride Equation
Produced hydrogen chloride vapor can behave as an acidic compound can release H ions in the water.
Ammonia and hydrogen chloride equation. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is a solid white smog. NH3HCl NH4Cl Convert 300 g of NH3 and 410 g of HCl to moles___ mol NH3 ___mol HCl Identify the limiting reactant when these quantities are mixed. In water the reaction between ammonia NH 3 and hydrogen chloride HCl is a textbook example of acid-base chemistry.
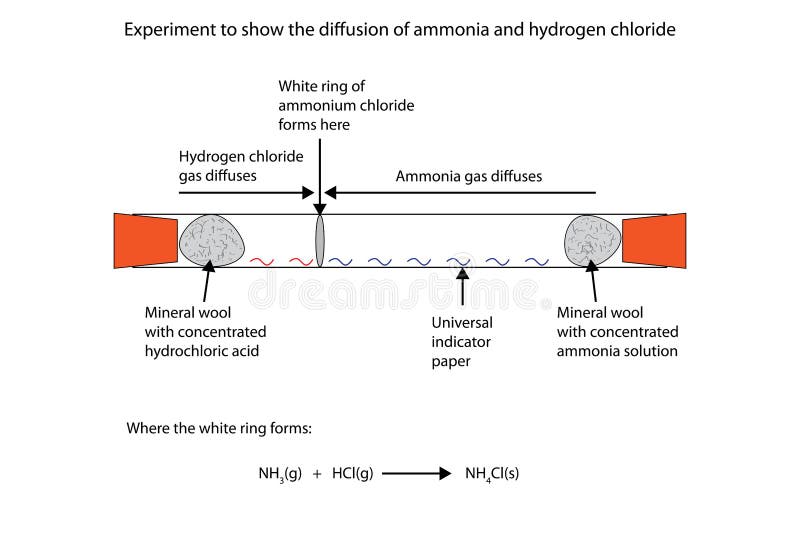
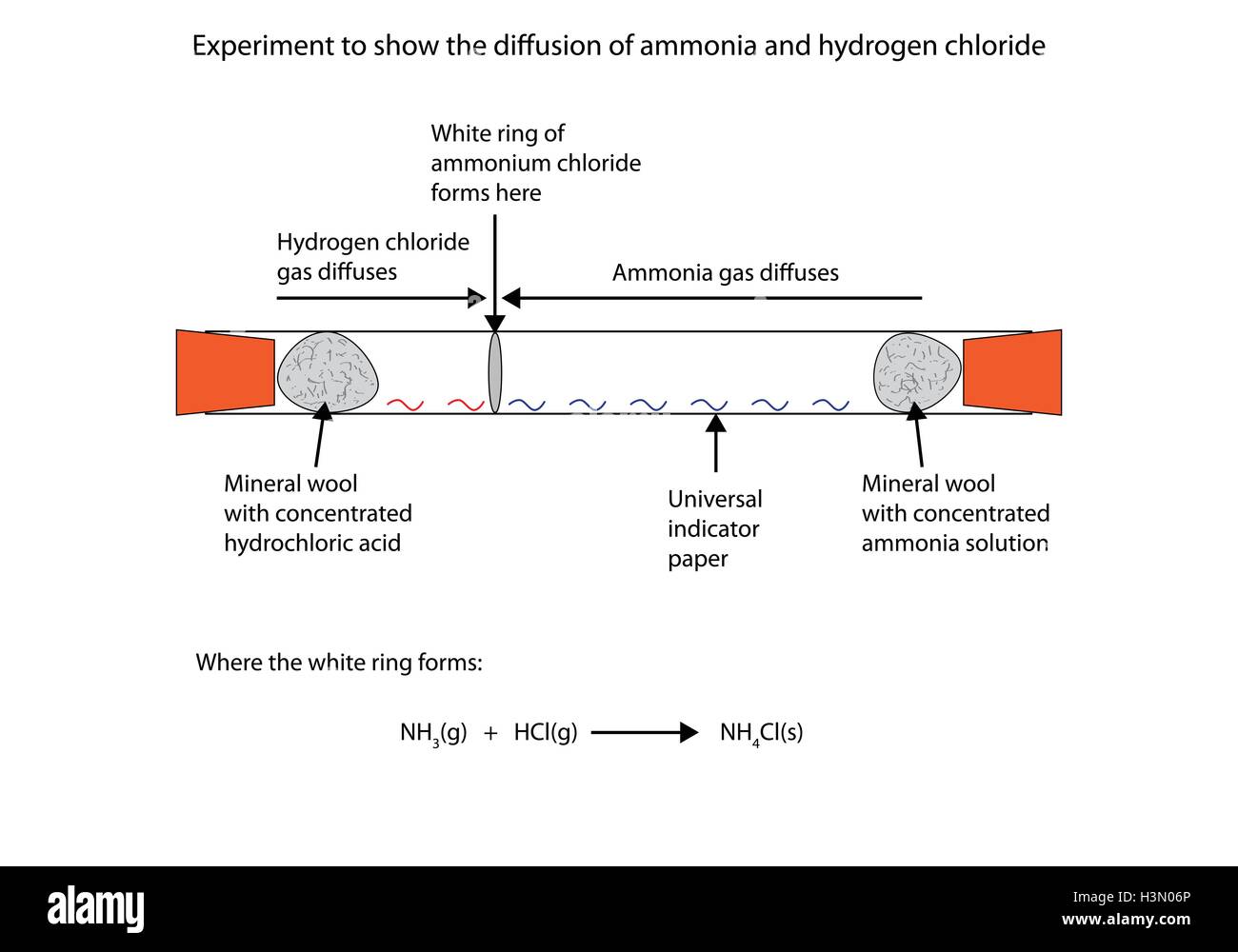
Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride. The ring of white powder is closer to the HCl than the NH 3. Ammonia NH 3 g and hydrogen chloride HCl g react to form solid ammonium chloride NH 4 Cl s.
The theft leaves chloride alone and negative. Write an equation for this reaction. In water the reaction between ammonia NH3 and hydrogen chloride HCl is a textbook example of acid-base chemistry most of us learn in high school.
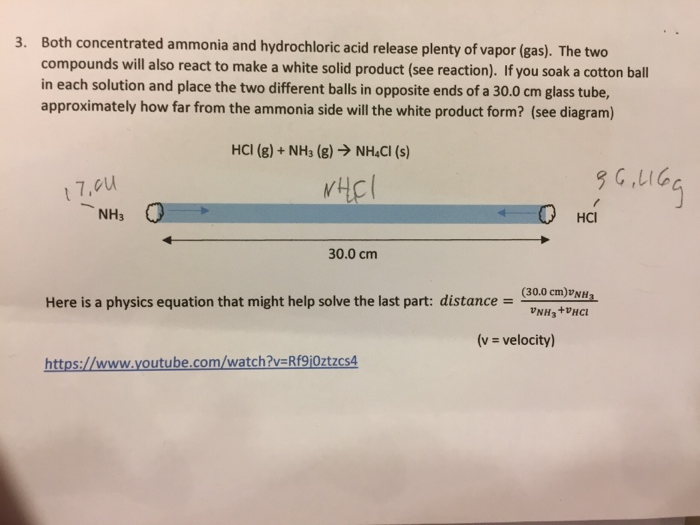
The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. NH 3 g HCl g NH 4 Cl s Two 250 L flasks at 300 o C are connected by a stopcock as shown in the drawing One flask contains 560g NH. Write the formula equation for the following reaction.
Ammonia reacts with hydrogen chloride to form ammonium loride. To happen this second step reaction ammonia is required. It breaks down when heated forming ammonia and hydrogen chloride.
The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. HCl g NH 3 g NH 4 Cl s The sign shows that the reaction is reversible. By its chemical nature the nitrogen in ammonia prefers to be attached to four hydrogens rather than the mere three it has so it steals the hydrogen from hydrogen chloride.