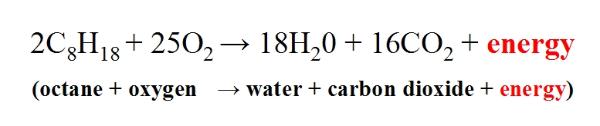Ideal Gasoline Burning Reaction

Burning gasoline may not seem like the kinds of chemical reactions that you have seen before.
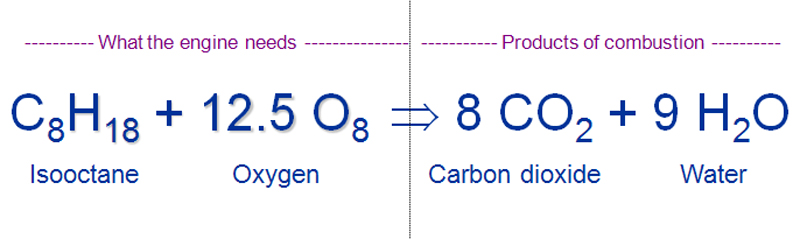
Gasoline burning reaction. Carbon monoxide is a colorless odorless gas that can be. Burning in the esophagus. Gasoline is usually approximated as being made up of only octane whose chemical formula is C8H18.
Gasoline is a toxic and highly flammable liquid. You are burning ethane. Fuel any hydrocarbon source plus oxygen yields carbon dioxide and water and energy.
When gasoline burns it doesnt seem to change into a. The oxygen atom will mix with the hydrogen and carbon atoms to form water and carbon dioxide source. Burning gasoline also produces carbon dioxide a greenhouse gas.
Under ideal settings where only hydrocarbon and oxygen are present the chemical reaction commonly called combustion or burning produces only water carbon dioxide and energy as the following basic equation shows. Burning gasoline releases several harmful chemicals one of which is carbon monoxide. Burning gasoline -When we burn gasoline we are combusting it or combining it with oxygen.
As the reaction equation illustrates carbon dioxide gas is produced when octane is burned. Thats why engines need a source of oxygen-containing air and why engines emit carbon dioxide as a by-product of combustion. SO x gases are formed when fuel containing sulfur such as coal and oil is burned and when gasoline is extracted from oil or metals are extracted from ore.
In the above ideal reaction the energy gained from the reaction is greater than the energy put into the reaction. Combustion or burning is a high-temperature exothermic redox chemical reaction between a fuel the reductant and an oxidant usually atmospheric oxygen that produces oxidized often gaseous products in a mixture termed as smoke. Remember the formula for sodium hypochlorite is NaClO so it can readily donate that oxygen atom to hydrocarbons.