Fabulous Ammonia Gas Balanced Equation

Give balanced chemical equations for the following.
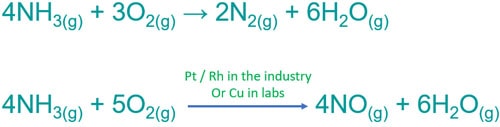
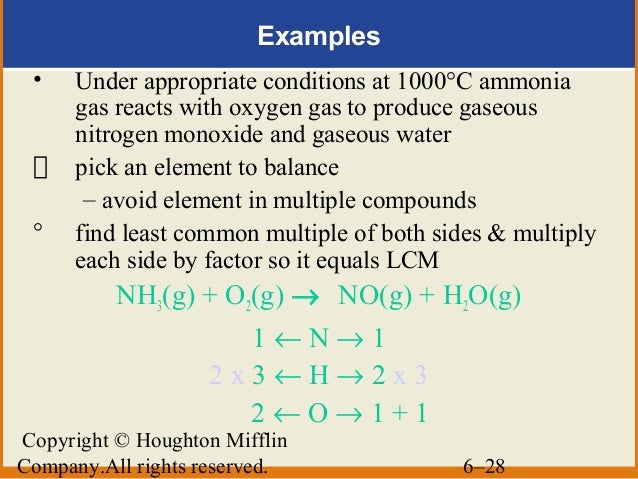
Ammonia gas balanced equation. This means that the lone pair is the negative end of a polar molecule. Balanced equation of NH 3 O 2 without catalyst 4NH 3 g 3O 2 g 2N 2 g 6H 2 O g Both ammonia and nitrogen gas are colorless gases. 2NH4Cl Ca OH2 CaCl2 2H2O 2NH3 b The ammonia gas is dried by passing through a drying tower containing lumps of quicklime CaO.
Here only chlorine atoms should be considered. Write the balanced chemical equation to prepare ammonia gas in the laboratory by using an alkali. Use uppercase for the first character in the element and lowercase for the second character.
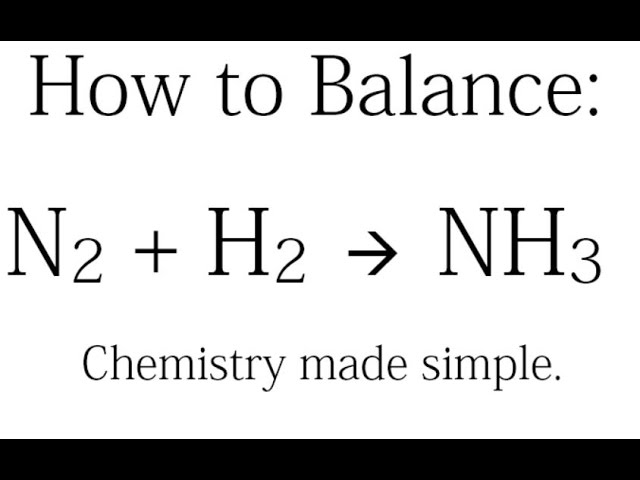
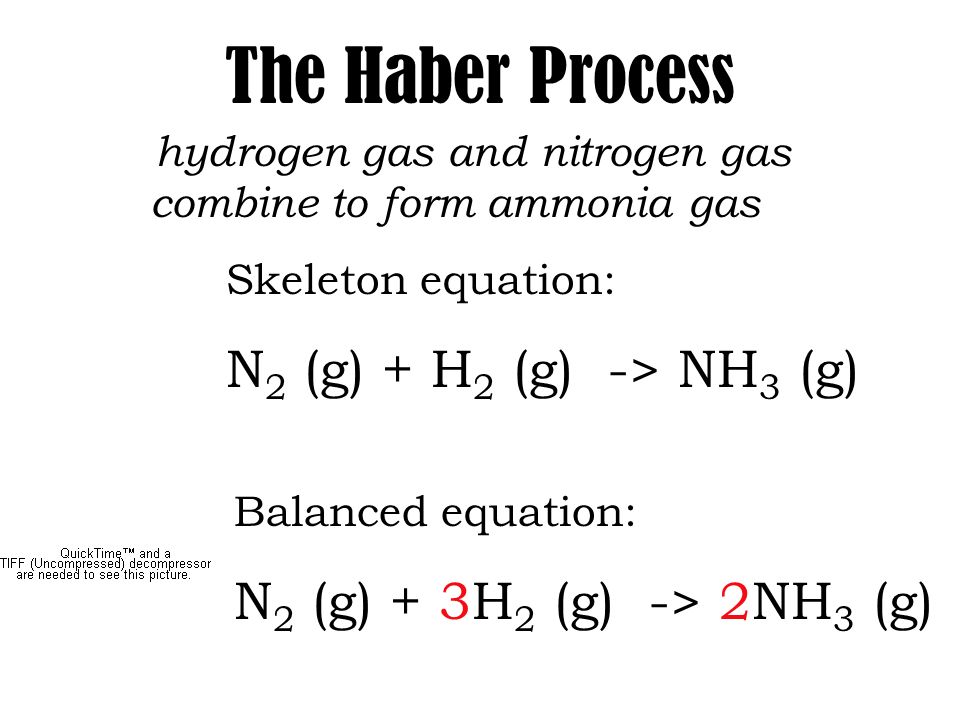
In this video well balance the equation NH3 O2 N2 H2O and provide the correct coefficients for each compoundTo balance NH3 O2 N2 H2O youll nee. The balanced equation for the reaction of nitrogen and hydrogen that yields ammonia is N2 3H2 produces 2NH3. According to the a balanced reaction equation 1 mole of ammonia gas reacts with exactly 1 mole of hydrogen chloride gas to produce the solid ammonium chloride.
Nitrogen gas is evolved during this reaction. Fe Au Co Br C O N F. Ammonia is a compound that is colorless and has a pungent odor.
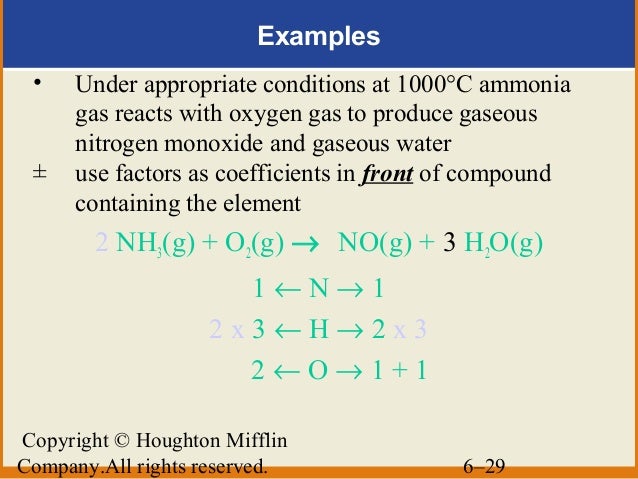
Therefore it is not collected over water. You see only oxidation number of chlorine atoms are changed while nitrogens and hydrogens are kept same in left side and right side. With supply of heat ammonia reacts with oxygen and produce nitrogen gas and water as products.
Balanced equation of NH 3 O 2 without catalyst 4NH 3 g 3O 2 g 2N 2 g 6H 2 O g. 3H2 N2 2NH3. This equation means that it requires one molecule of nitrogen gas to react with three molecules of hydrogen gas to form two molecules of ammonia.