Perfect Butane Oxygen Reaction

To create a proper balance one may only adjust the numbers of.
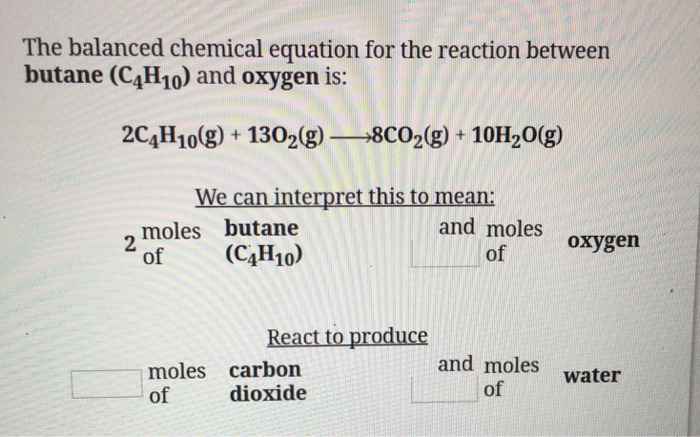
Butane oxygen reaction. When there is sufficient oxygen. Carbon dioxide and water. The combination produces eight molecules of carbon dioxide and 10 water molecules.
When oxygen is limited carbon soot or carbon monoxide may also be formed. Moles of carbon dioxide and moles of water The balanced chemical equation for the reaction between propane C3Hg and oxygen is. Click card to see definition 22 moles.
It can also react with oxidizers. 2 C4H10 g 13 O2 g - 8 CO2 g 10 H2O l In a particular reaction 500 moles of C4H10 were reacted with an excess of oxygen O2. G of butane is mixed with 150.
The reaction between ethane and oxygen is a combustion reaction. This corresponds to an atmospheric half-life of about 63 days SRC at an atmospheric concentration of 5X105 hydroxyl radicals per cu cm 2. What is the balanced equation when ethane reacts with oxygen.
Calculate the maximum mass of carbon dioxide. When butane burns in an excess of oxygen the products are. Butane is denser than air.
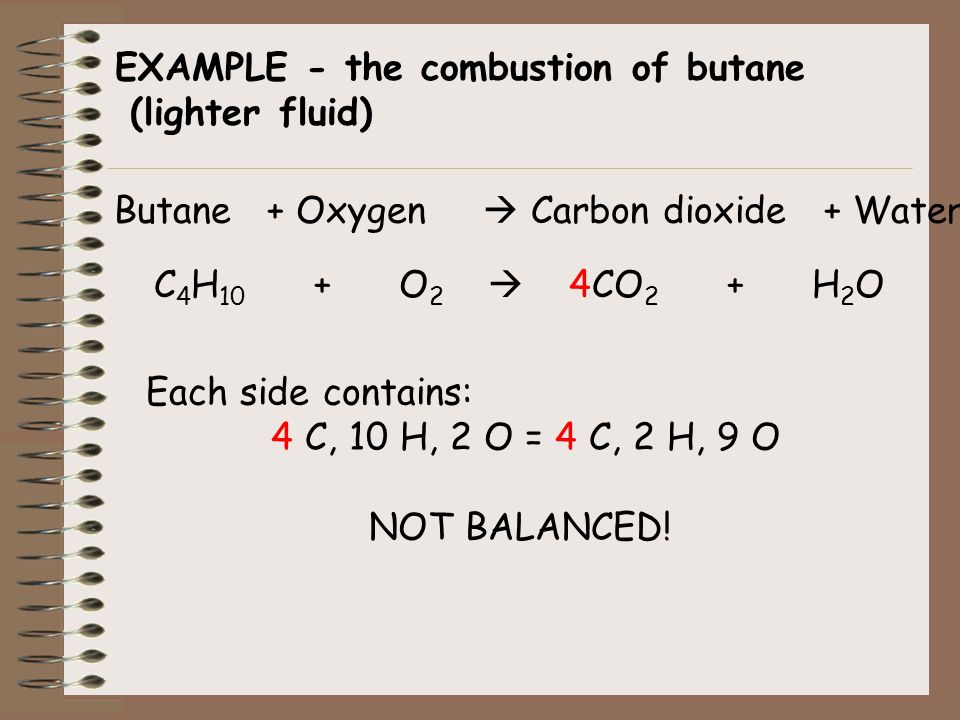
Gaseous butane will react with gaseous oxygen to produce Gaseous butane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water. And the mandatory products are carbon dioxide and water. 2 C4H10g 13 O2g 8 CO2g 10 H2Og We can interpret this to mean.