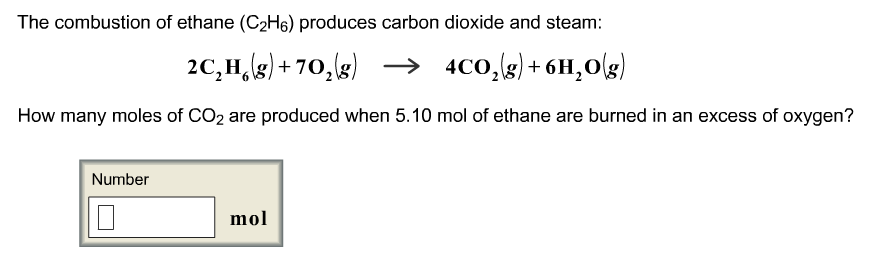Outrageous Balanced Combustion Of Ethane
They are scarce in nature.
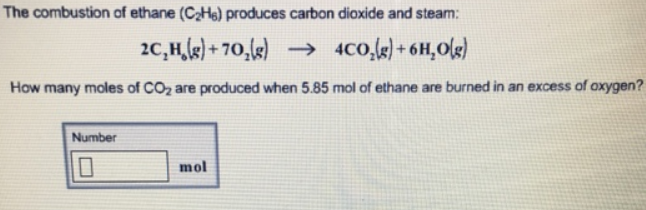
Balanced combustion of ethane. What is the heat of combustion of ethane-15595. Pha symbola and ene changes are optional. During the combustion of 500 g of ethane C2H6 355 kcal is released a Write a balanced equation for the combustion of ethane b What is the sign of H for this reaction.
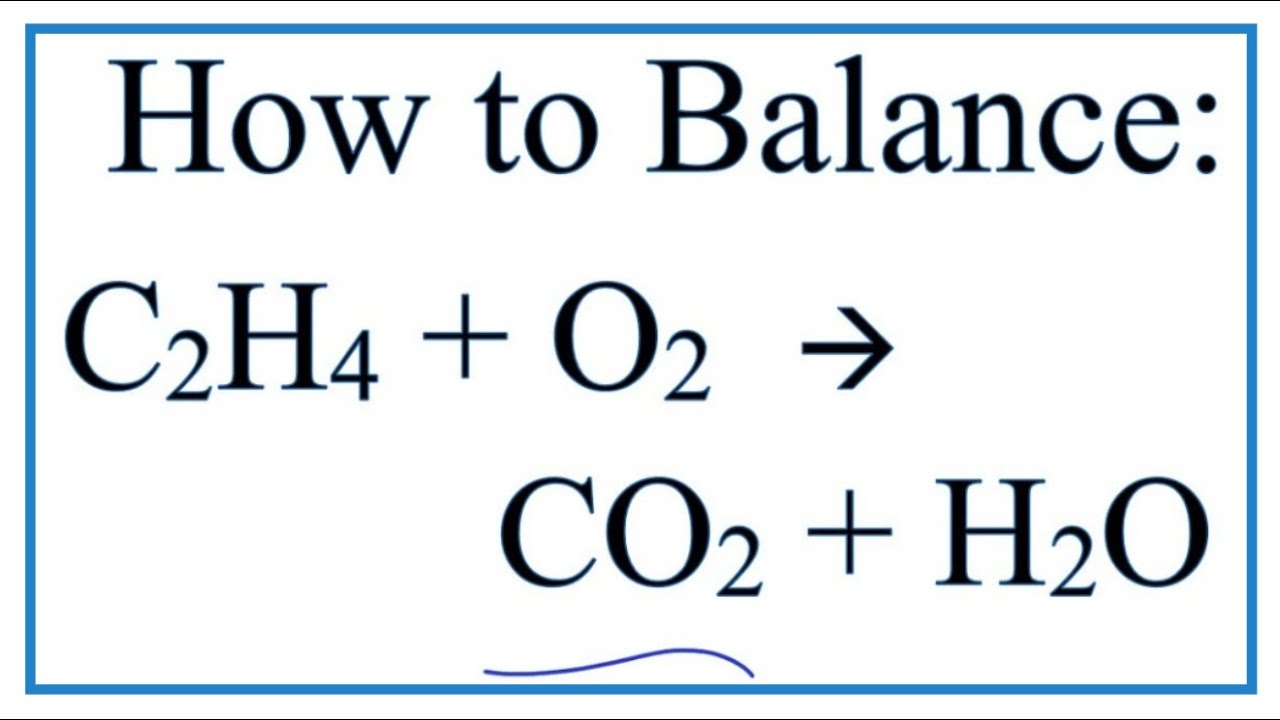
Methane burns readily in air. Balancing hydrocarbon combustion reactions can be tricky but if with practice they can be really fun and very rewarding. As there are 12 on the left but only 2 on the right we need to add a 6 in front of the H2O to produce 12 in total 2 x 6 12.
Its easier to leave the O2 to the last it has a way to alter the equation. Why are alkenes not used as fuels. Fuel O2 CO2 H2O You would then balance the chemical equation.
Fe Au Co Br C O N F. Alkenes are not used as fuels because. Dioxygen - O 2.
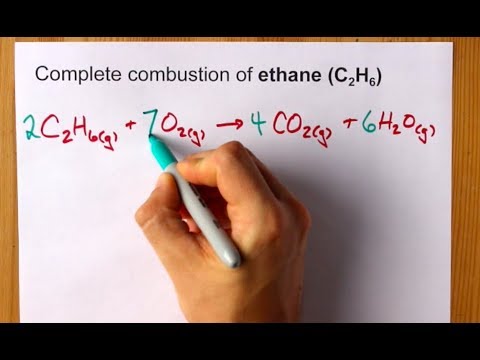
C2H6 O2 Co2 H2O. Is it possible to isolate pure staggered ethane or pure eclipsed ethane at room temperature. What is the balanced equation of Ethane.
In Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions. 2 C 2 H 6 7 O 2 4 CO 2 6 H 2 O Heat Energy Enthalpy The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. Ethane C2H6 reacts with oxygen O2 to make carbon dioxide CO2 and water H2O.