Smart Ethane Combustion Equation Balanced

Write a balanced equation for the complete combustion of ethane with oxygen.
Ethane combustion equation balanced. Fuel O2 CO2 H2O You would then balance the chemical equation. A combustion reaction has a general reaction of. In a combustion reaction with a hydrocarbon in the reactant side you will always have O2 as another reactant.
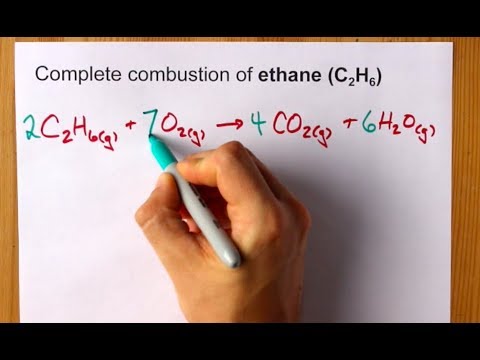
2 C 2 H 6 7 O 2 4 CO 2 6 H 2 O Heat Energy Enthalpy The hydrocarbon combustion reaction releases heat energy and is an example of an exothermic reaction. The balance equation for the complete combustion of ethane. The process of combustion taking place in ample supply of oxygen is known as complete combustion.
Click hereto get an answer to your question The balance equation for the complete combustion of ethane is. Ethane has a chemical formula of C2H6. The reaction also has a negative enthalpy change ΔH value.
As you will always have CO2 and H 2O as the products. C2H6 O2 Co2 H2O. Write and balance the equation for the complete combustion of ethane C2H6.
The balanced chemical equation for the complete combustion of ethane is. During the combustion of 500g of octane C8H18 2395 kcal is released. What would be the balanced chemical equation for the complete combustion of pentane C5H12.
2C2H6 g7O2 g--4CO2 g6H2O g If the ethane is burning at the rate of 07 molL s at what rates are and being produced. What is the balanced equation of Ethane. Ethane is an alkane with the chemical formula C2H6.