Favorite How To Calculate Exothermic Reaction

Examples of exothermic reactions are combustion burning and neutralisation.
How to calculate exothermic reaction. These are exothermic reactionsExothermic reactions may occur spontaneously and result in higher randomness or entropy ΔS 0 of the system. A few examples are neutralization burning a substance reactions of fuels deposition of dry ice respiration solution of. Energy is given off as.
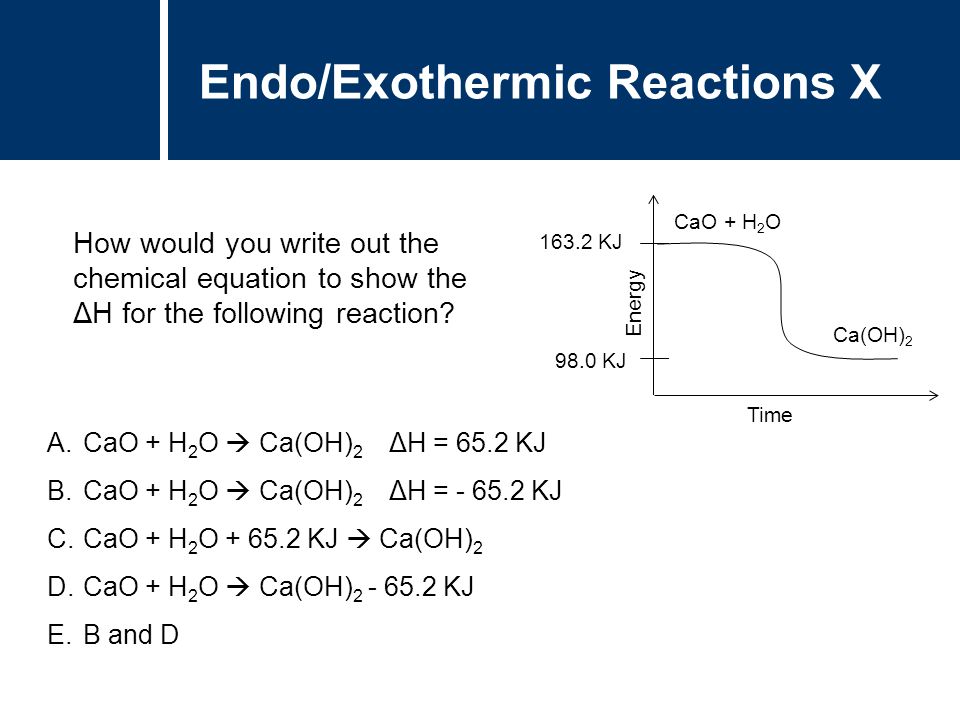
This means heat was released to the surroundings or that the environment gained energy. Calculate the Delta H for the reaction and write whether the final Delta H is exothermic or endothermic beginmatrix 4NH_3. The exothermic reaction is the opposite of an endothermic reaction.
Calculate Rate Of Reaction For Highly Exothermic Semi Batch Reaction - posted in Industrial Professionals. Any increase in temperature indicates an exothermic reaction. Reactants Products Energy What is an Exothermic Reaction.
When water becomes a solid it releases heat warming up its surroundings. They are denoted by a negative heat flow heat is lost to the surroundings and decrease in enthalpy ΔH 0. Note the increase in the surrounding entropy since the reaction was exothermic.
If the products side has a larger enthalpy the reaction is endothermic. Is the freezing of water exothermic. With H a scientist can determine whether a reaction gives off heat or is exothermic or takes in heat or is endothermic.
A negative value indicates the reactants have greater enthalpy or that it is an exothermic reaction heat is produced º signifies that the reaction is a standard enthalpy change and occurs at a preset pressuretemperature rxn denotes that this change is the enthalpy of reaction. The reaction is exothermic. An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light.