Neat Copper Sulfate And Hcl
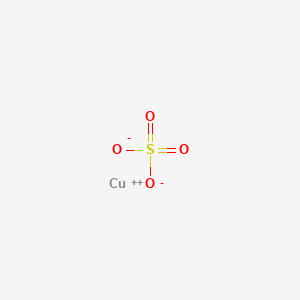
Typically sulphate or chloride.
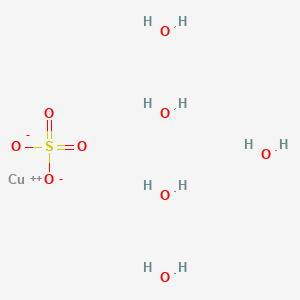
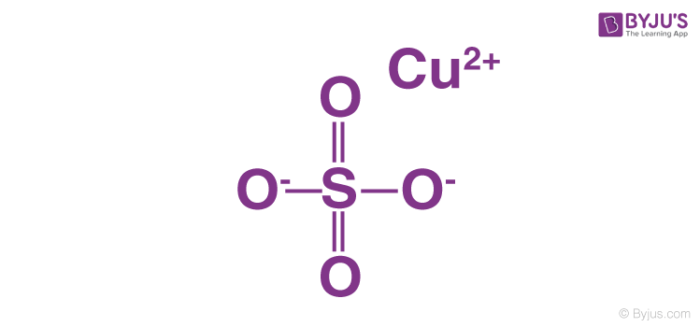
Copper sulfate and hcl. Next is it in crystalline form or a saturated solution. Copper II Sulfate and Hydrochloric Acid react to yield Hydrogen Sulfate and Copper II Chloride. Here hydrochloric acid HCl is added to copper II sulfate CuSO 4.
CuSO 4 nH 2 O. The reversible copper sulfate reaction When concentrated hydrochloric acid is added to a very dilute solution of copper sulfate the pale blue solution slowly turns yellow-green on the formation of a copper chloride complex. Copper sulphate react with sodium carbonate and water to produce copper hydroxocarbonate sodium sulfate and carbon dioxide.
It contains a copper II sulfate. In this reaction the element zinc replaced copper in the compound copper sulfate thus creating zinc sulfate. There are very strong odds that it is the hydrated form which is what nearly everyone means when they say copper sulfate.
101 Copper oxide CuO or CuII differs from cuprous oxide Cu 2O or CuI by coppers oxidation status. Duration 73 seconds size 427 K file name MOVIESCUSOCUSOHCLXMOV. Copper sulfate is highly soluble in water with solubility values of 1055 molal and 1502 molal ate 10 o C and 30 o C respectively.
The pentahydrate CuSO 4 5H 2 O the most commonly encountered salt is bright blue. It contains a copper 2. This is a classic displacement reaction.
The solution can change color several times by alternating the addition of water and hydrochloric acid. Now you will need a good chemistry book to see if there is any reaction. The release of hydrogen gas can be tested using a burning splinter for a pop-sound.