Nice Ammonia Reacts With Hydrogen Chloride Gas

The wizard gives Rick the task of carrying water from a well to the garden.
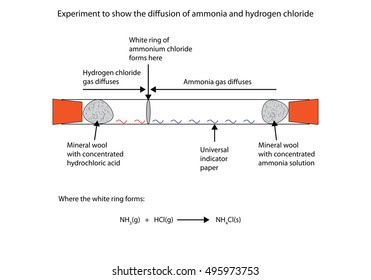
Ammonia reacts with hydrogen chloride gas. 2 stoppered Erlenmeyer flasks with ammonia and hydrogen chloride gases are opened and the product of the reaction ammonium chloride white smoke will appea. Here only chlorine atoms should be considered. The volume occupied by NH3 in glass bulb A is three times more than the volume occupied by HCl in glass bulb B at STPi How many moles of ammonia are present in glass bulb A ii How many grams of NH3Cl will be formed when the stopper is opened.
Reaction with oxidizers such as permanganates chlorates chlorites and hypochlorites may produce chlorine or bromine gas. D Potassium metal react with water. Ammonium chloride which is.
Hydrochloric acid and hydrogen chloride react violently with many metals with the generation of highly flammable hydrogen gas which may explode. According to the a balanced reaction equation 1 mole of ammonia gas reacts with exactly 1 mole of hydrogen chloride gas to produce the solid ammonium chloride. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which.
NH3 g HCl g NH 4Cl s Two 250 L flasks at 300 oC are connected by a stopcock as shown in the drawing One flask contains 560 g NH3 g and the other contains 460 g HClg. A Hydrogen gas combines with nitrogen to form ammonia. The theft leaves chloride alone and negative.
This is used as a test to detect the presence of ammonia gas as per the reaction. N H 3 gH C lg N H 4. Agent Material Selection 4 Ratings given are based at 70F 21C.
The system uses ammonia water and hydrogen in closed loop system. After the reaction occurs and the temperature returns to 22degreeC what is the pressure inside the flask. When ammonia reacts with hydrogen chloride gas it produces white fumes of ammonium chloride.