Fun Define Chemical Displacement

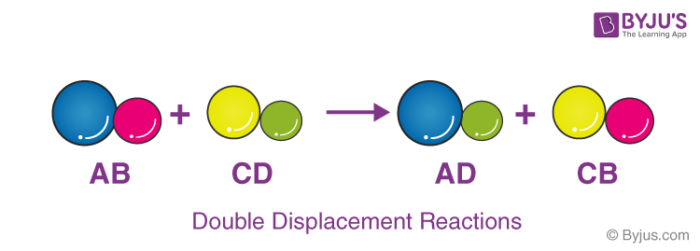
A double displacement reaction is a type of chemical reaction in which the reactant ions exchange places to form new products.
Define chemical displacement. There are two types of displacement reactions. Encyclopedia article about Chemical displacement by The Free Dictionary. The basis for different types of reactions is the product formed the changes that occur the reactants involved and so on.
A displacement reaction is a type of reaction in which part of one reactant is replaced by another reactant. Double-displacement meaning A chemical reaction between two compounds in which the first and second parts of one reactant are united respectively with the second and first parts of the other reactant. Substitution reaction n reazione f di sostituzione.
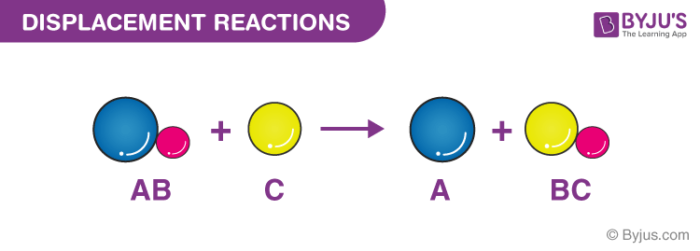
A displacement reaction is a chemical reaction that occurs by replacing a chemical species in a molecule by another chemical species. In single displacement reactions the more reactive element displaces the less reactive one. Reaction of iron nails with copper sulphate solution.
Both metals and non-metals take part in displacement reactions. A displacement reaction is also known as a replacement reaction or a metathesis reaction. In a displacement reaction.
It is also called a replacement reaction. Those reactions in which one element takes place of another element in a compound are known as substitution reactions. Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound.
Displacement Reaction - definition Displacement reaction is a chemical reaction in which a more reactive element displaces a less reactive element from its compound. The chemical bonds between the reactants may be either covalent or ionic. Displacement Reaction Or Substitution Reaction.