Fantastic Ethane Reacts With Oxygen Balanced Equation

And the mandatory products are carbon dioxide and water.
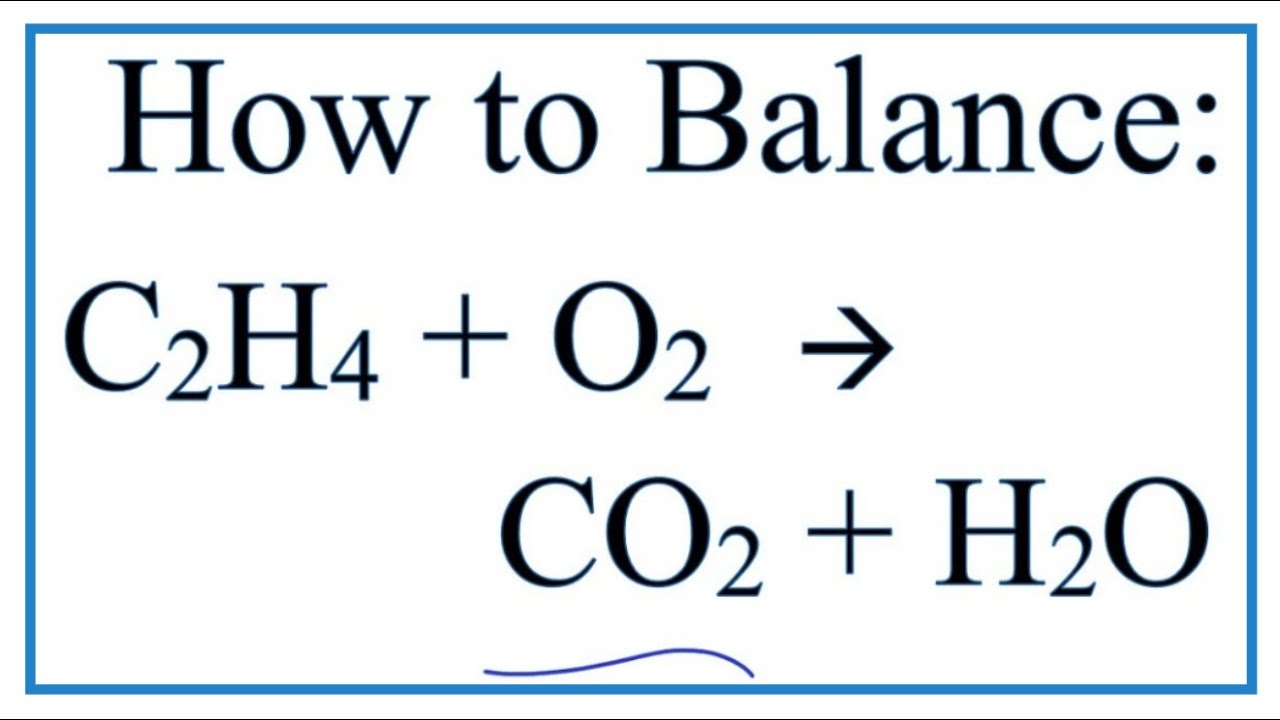
Ethane reacts with oxygen balanced equation. Ethane reacts with excess oxygen to produce carbon dioxide and water. Find an answer to your question When the equation for the combustion of ethane C2H6 is correctly balanced the coefficient on oxygen is. Ionic charges are not yet supported and will be ignored.
How many moles of O2 are needed to completely combust 250 moles of ethane. Ethane gas C2H6 C 2 H 6 reacts with oxygen gas to produce carbon dioxide gas and water vapor according to the reaction shown below. So in a combustion reaction it is sure that one of the reactants will be oxygen.
The reaction between ethane and oxygen is a combustion reaction. Adding HCl to ethene to form chloroethane. In the presence of sufficient oxygen LPG burns to form water vapour and carbon dioxide as well as heat.
A metal x with relative atomic mass of 56forms an oxide with formul X2O3How many grams of the metals will combine with 10g of oxygen Ethane burns completely in oxygen according to the equations below C2H672O22CO 3H2O what. Ethanol C2H5OH burns with oxygen in air to give carbon dioxide and water. The balanced equation will appear above.
Like how how margarine is made when. Addition reactions are when we are adding species to another compound eg. In Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions.
If not enough oxygen is present for complete combustion of LPG incomplete propane combustion occurs with water carbon dioxide and carbon monoxide being produced. Ethane is an alkane with the chemical formula C2H6. Use uppercase for the first character in the element and lowercase for the second character.