Cool Ethane Water Reaction
Steam reforming or steam methane reforming is a method for producing syngas hydrogen and carbon monoxide by reaction of hydrocarbons with water.
Ethane water reaction. Balance the equation and calculate enthalpy change eqDelta H eq for the reaction. Epoxyethane reacts with water in the presence of an acid catalyst very dilute sulphuric acid at a temperature of about 60C. When atoms are added to a monomer or polymer this is called an ADDITION REACTION.
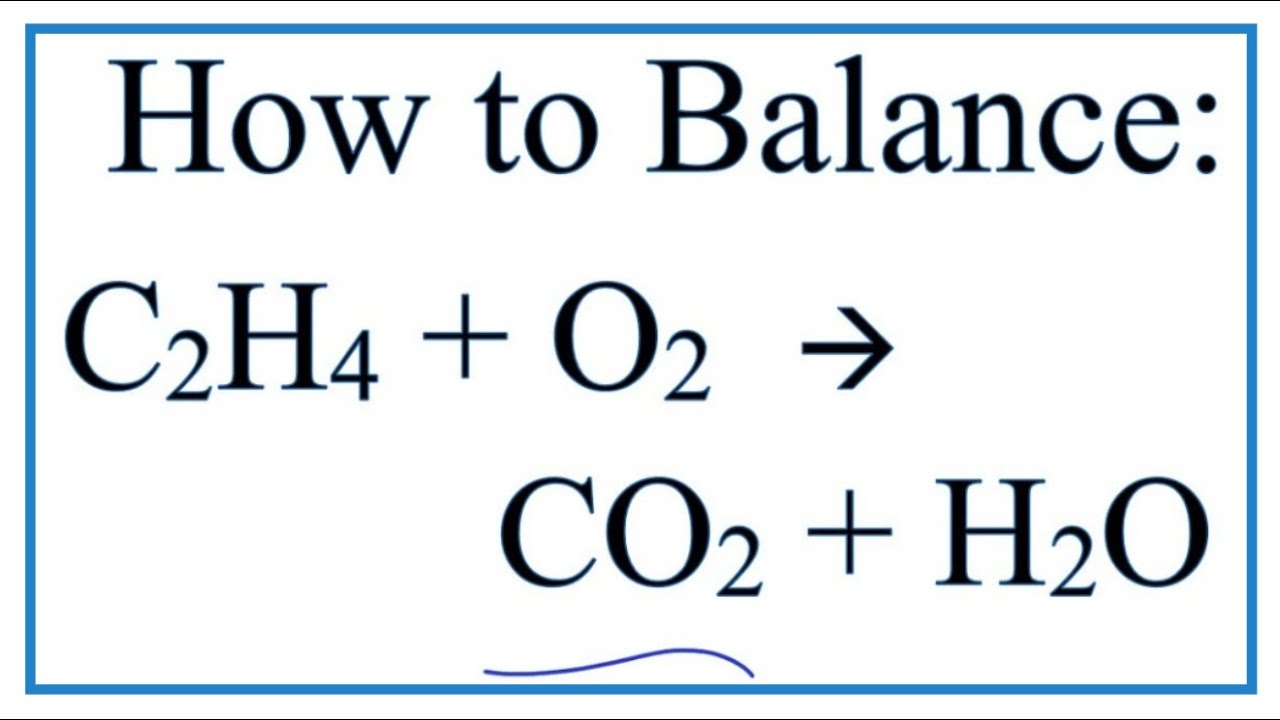
Gaseous ethane CHCH reacts with gaseous oxygen gas 0 to produce gaseous carbon dioxide CO and gaseous water H0. However there is an interaction. C 2 H 5 Cl 2 C 2 H 5 Cl Cl.
Water is formed at a rate equal to two-thirds the rate of formation of CO 2. CH 4 H 2 O CO 3 H 2. Ethane CH 3 CH 3 being just an alkane will not react with the water or the acid as ethane has no reactive sites.
In both cases carbon dioxide and water are produced. Ethylene CH 2 CH 2 having an electrophilic double bond will react with water in the presence of an acid catalyst to form ethanol if Im not mistaken. The rate of consumption of ethane is seven times faster than the rate of consumption of oxygen.
The rate of formation of CO never changes. Ethane C_2H_6 reacts with molecular oxygen to produce carbon dioxide and water. The dehydrogenation of ethane to ethylene in the presence of oxygen and water was conducted using Na 2 WO 4 SiO 2 catalyst at high temperatures.
Ethane can react with the halogens especially chlorine and bromine by free radical halogenation. Epoxyethane reacts with water in the presence of an acid catalyst very dilute sulphuric acid at a temperature of about 60C. Chemistry Chemical Reactions Balancing Chemical Equations.