Cool Formation Of Aluminium Oxide

Moreover it happens to be the most commonly occurring of the aluminium oxides.
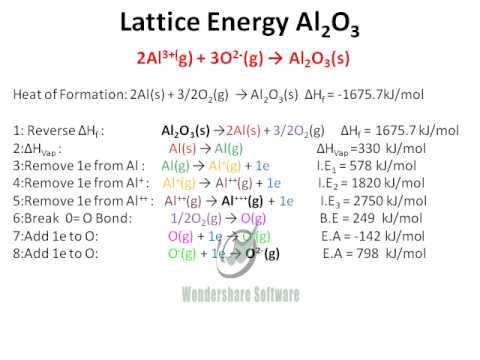
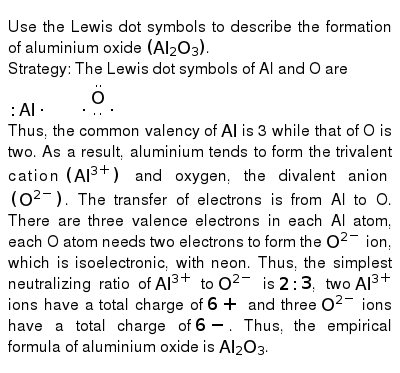
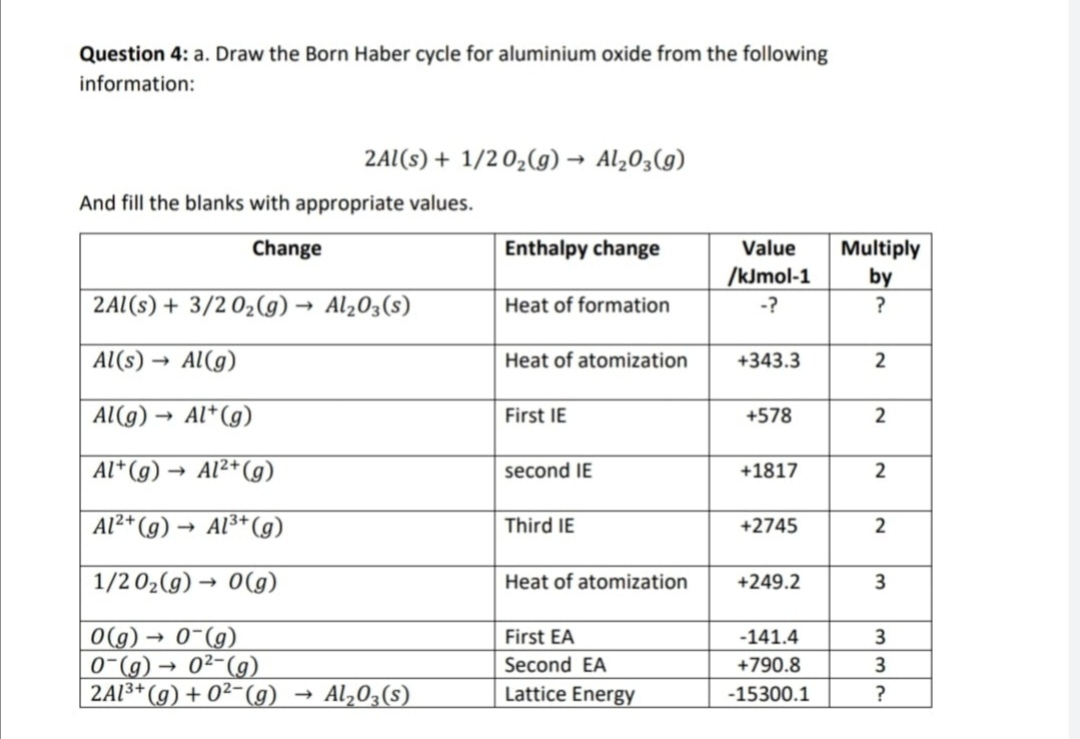
Formation of aluminium oxide. The aluminum oxide also called alumina aluminum oxide corundum or aluminum trioxide is an inorganic compound whose chemical formula is the A 2 O 3. The kinetics of anodic oxide film formation are governed by 1 the thermodynamics at the surface and 2 diffusion and mass transfer across the oxide layer as it forms. 4 Al s 3 O2 g 2 Al2O3 s Write the six mole ratios that can be derived from this equation.
However when bonded with oxygen to form aluminium oxide a protective coating forms and prevents further oxidation. This results in two triple positive aluminium ions to. The molecule formation takes place with 3 hydroxyl anions or CO3-2 and aluminium cation or Al3.
The growth kinetics and mechanisms of thin aluminum-oxide films formed by the dry thermal oxidation of a bare Al 431 substrate at a partial oxygen pressure of 13310 4 Pa in the temperature range of 373773 K were studied using x-ray photoelectron spectroscopy. Aluminium hydroxide structure is completely based on the mineral from which it is extracted mainly because the ions tend to show varied arrangements. Inhalation exposure to 100 mghr aluminium in the form of powder or 92 mg Al per 2 hr as a fume each day for 9-13 months showed a significant retention of aluminium in the lungs of both groups of animals.
Two separate groups of pillar samples were fabricated via focused ion beam FIB micromachining each on an aluminium. It is an amphoteric oxide although it can become due to treatment an almost inert compound. Furthermore it is also known as alumina.
Majority of the mineral lattice is orthorhombic or hexagonal. However the following also applies. The oxide of aluminium is amphoteric acting primarily as a base Srivatrava and Farber 1978.
Emphasis is placed on the proposed mechanisms of scale growth based on observations of scale morphologies and microstructures inert-marker experiments and the distribution of oxygen isotope tracers within thermally-grown oxides. These properties among others have allowed aluminum oxide. When welding aluminium materials aluminium oxide is formed from the filler material and the base material.