Heartwarming Potassium Hydroxide And Water Balanced Equation

This equation does not have any specific information about phenomenon.
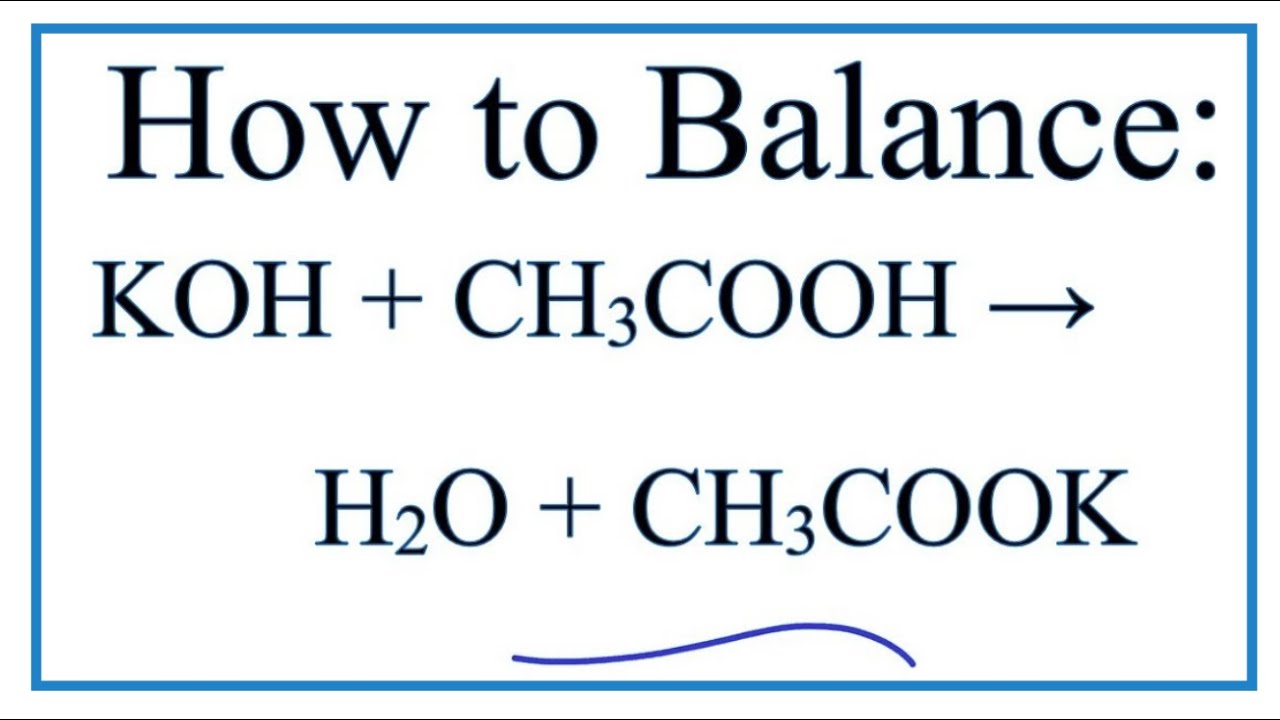
Potassium hydroxide and water balanced equation. Acid base Salt Water. Strong bases A strong base is something like sodium hydroxide or potassium hydroxide which is fully. K2OH2O2KOH The chemical reaction that takes place when potassium hydroxide pellets KOH is dissolved in water is called solvationKOH completely dissociates in water to its constituent ions.
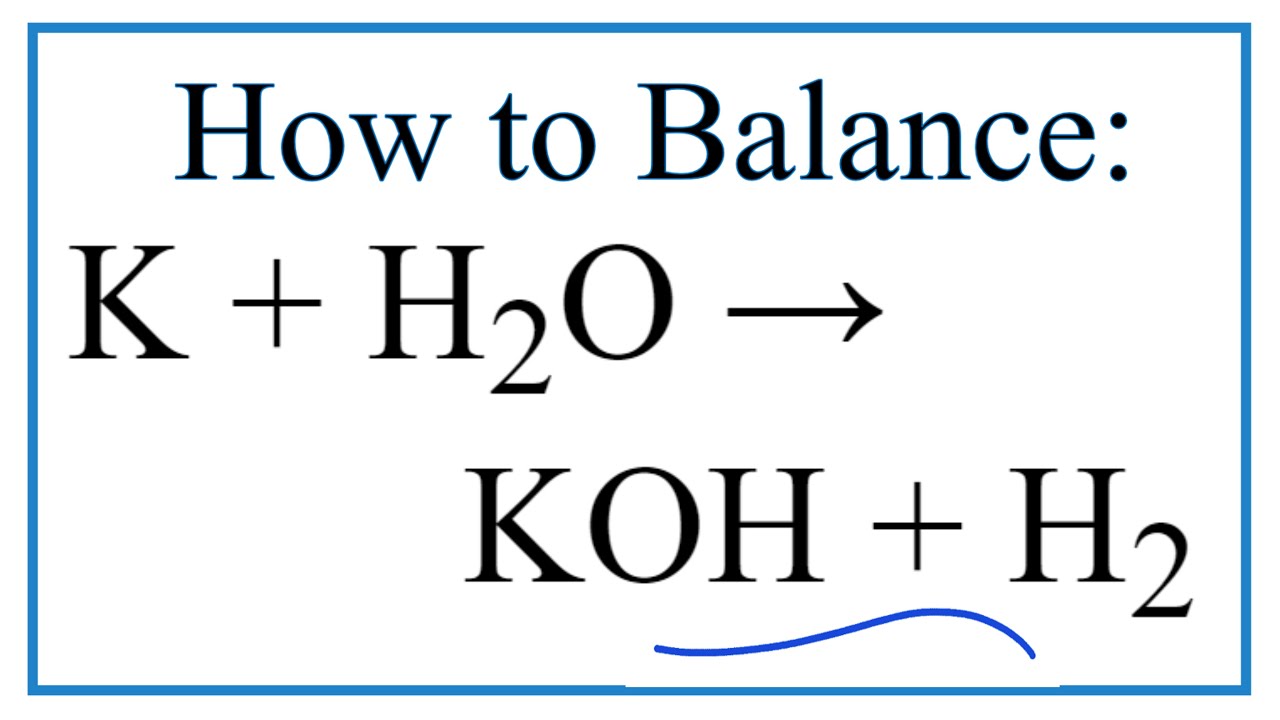
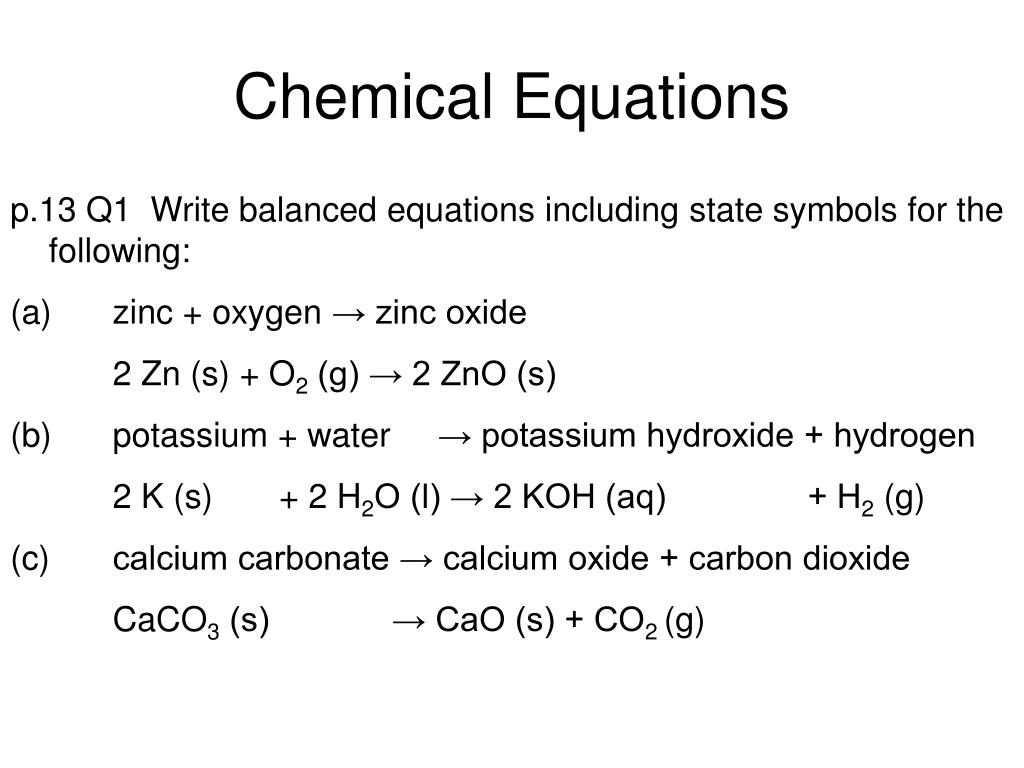
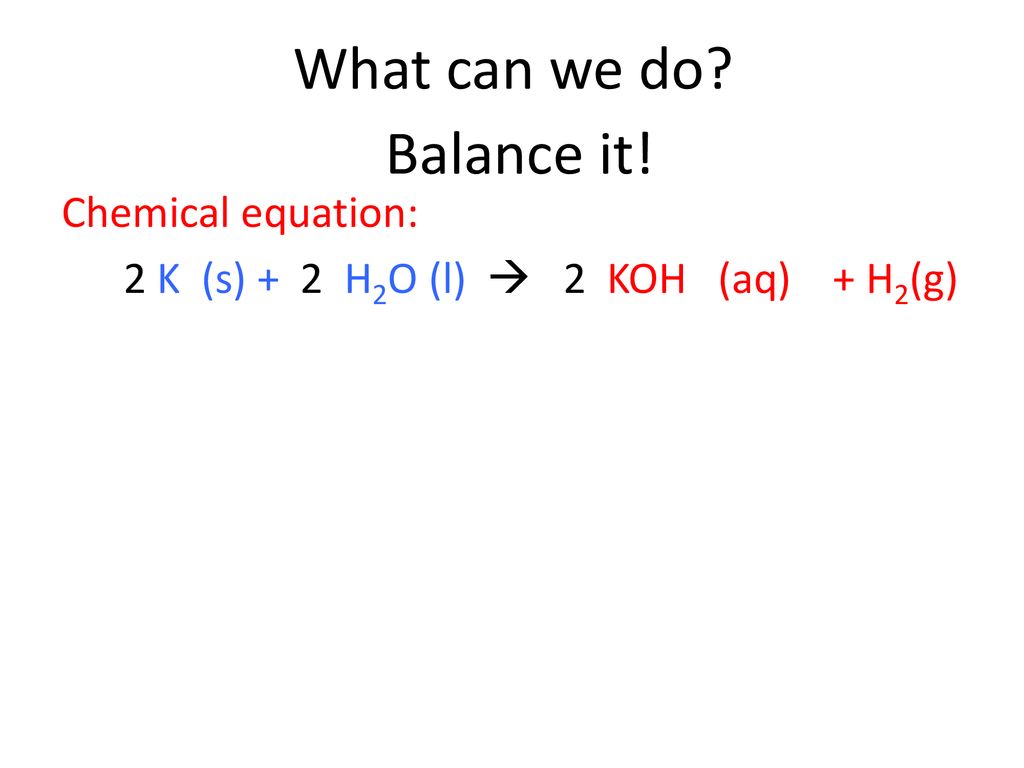
Hydrogen exists naturally as a diatomic molecule and is usually a gas at room temperature so it is written as H 2 g. Hydroiodic acid reacts with potassium hydroxide to form potassium iodide and water formed as a by-product. 2K s 2H 2 O l 2KOH aq H 2 g Potassium K is a group 1 metal which is a solid.
O P2SO4 2H20 1. K2O H2O KOH To balance the equation we place a coefficient of 2 in front of the potassium hydroxide. When sodium hydroxide is dissolved in water it completely dissociates into sodium and hydroxide ions.
In this case you just need to observe to see if product substance K3CrOH6 Potassium hexahydroxochromate III appearing at the end of the reaction. The unbalanced equation is. Chemistry questions and answers.
In addition when a saltcompound dissolves in water it BRE. The question is on a neutralization reaction taking place between potassium hydroxide and phosphoric acid which produces potassium phosphate and water only. H2SO4 2POH - P2SO4 2H20 1.
In this video well balance the equation Potassium hydroxide Nitric Acid and provide the correct coefficients for each compoundTo balance KOH HNO3 KN. For every mole of KOH there will be 1 mole of OH- so the concentration of OH- will be the same as the concentration of KOH. Write a balanced equation for the reaction of butanoic acid with potassium hydroxide and with water 2 separate reactions 23.