Formidable Incomplete Combustion Of Heptane
First be sure to count all of C H and O atoms on each side of the.
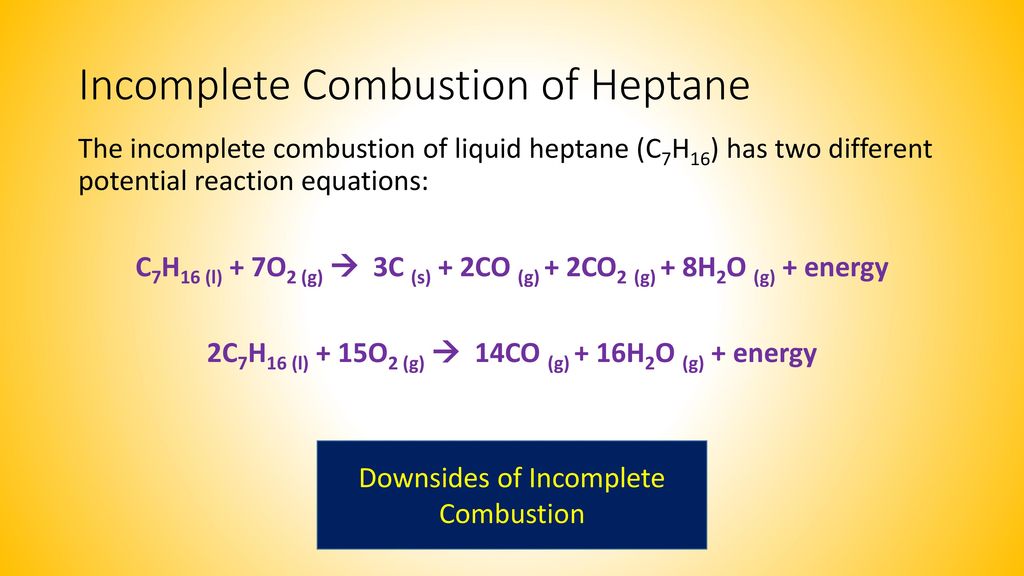
Incomplete combustion of heptane. Click for Incomplete Combustion of Heptane. Incomplete combustion often occurs in uncontrolled or less than ideal conditions. Heptane is also produced commercially by fractional distillation of hydrocarbon feedstock.
Heptane is metabolized to its parent alcohols mainly 2-heptanol and 3 heptanol and to a minor extent 1-heptanol and 4- heptanolThe heptanol metabolites are conjugated by glucuronates or sulfates and subsequently excreted in urine. Reactant O2 -- CO H2O. 9 rows for heptane.
The formula for heptane is C7H16. For example the incomplete combustion of heptane C 7 H 16 has two different potential reaction equations. Heptane is further metabolized at relatively high rates by hydroxylation before being converted to the corresponding keto forms.
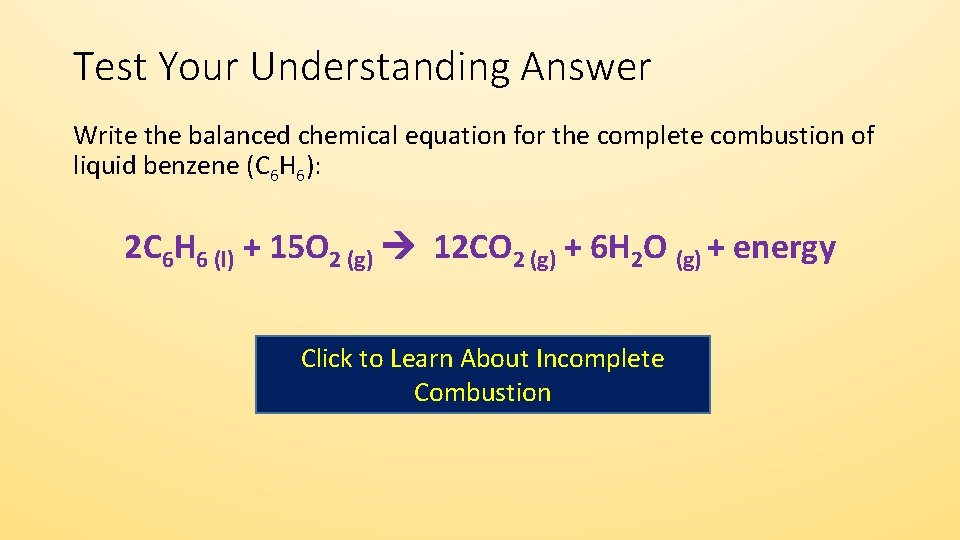
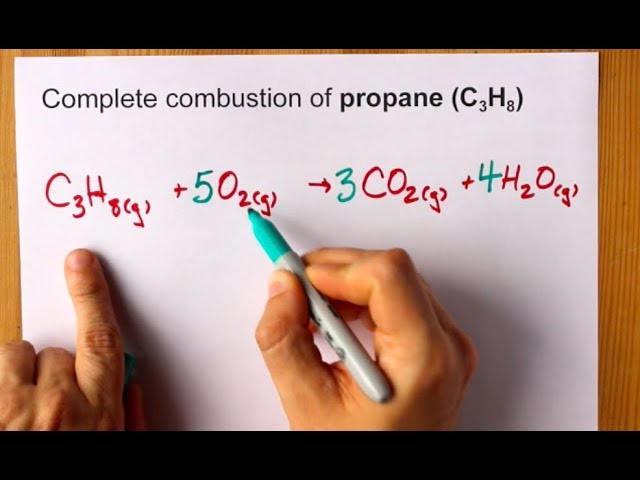
Then write the chemical equation for the complete combustion of heplane. C5H12 55 02 -- 5 CO 6 H20 So first write out your hydrocarbon 02 and then write CO2CO H20 according to whether it is complete or incomplete combustion the. Heptane can be released into the air from its production and use in many products associated with the petroleum and natural gas industries.
This will be seen with a more yellow flame. INCOMPLETE COMBUSTION The products of incomplete combustion include carbon dioxide and water vapour as well as carbon carbon monoxide or both Incomplete combustion is a more inefficient process for generating heat since it has less oxygen and therefore more light is produced rather than heat. Fossil fuel air pollution - incomplete.
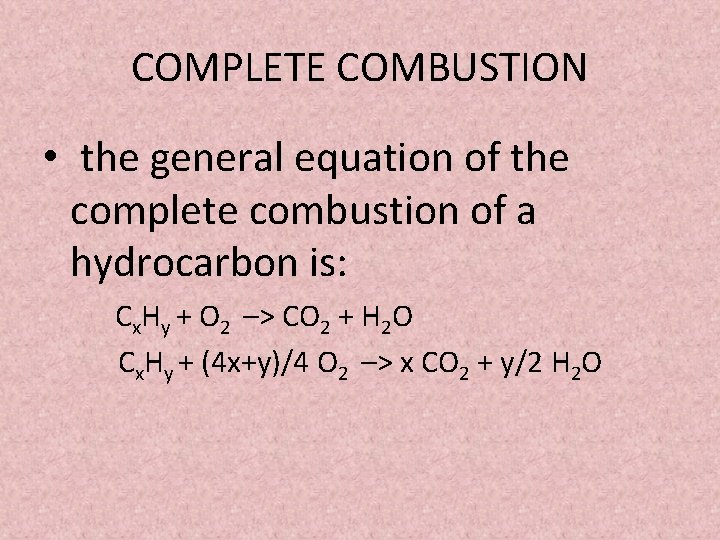
Start with the unbalanced equation. Incomplete combustion just means that instead of your reactant in this case heptane gas reacting with oxygen gas to form carbon dioxide and water it forms carbon monoxide and water. Compare and contrast these chemical equations.