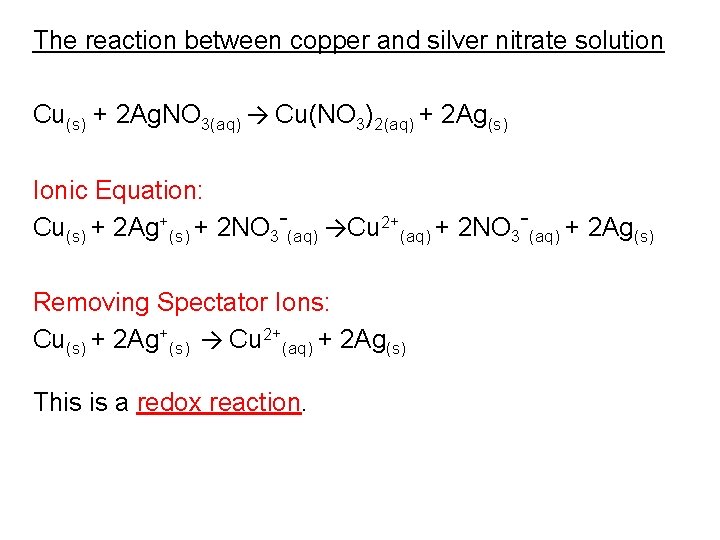
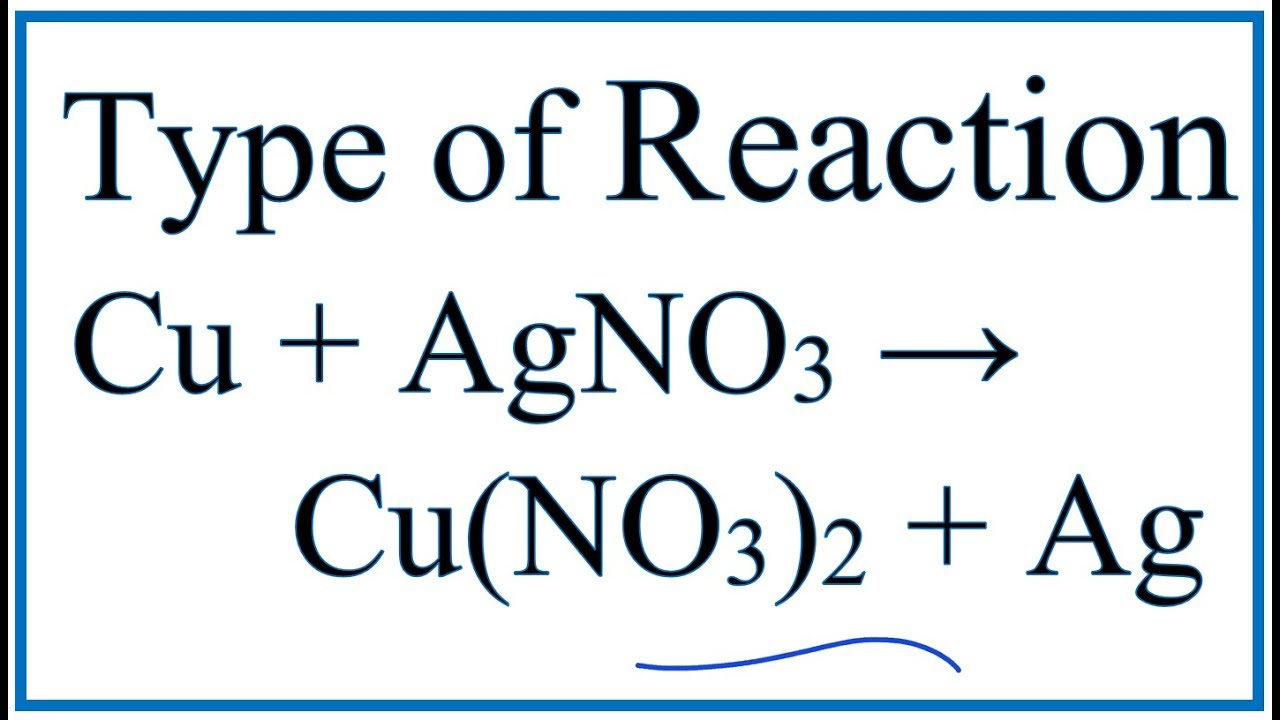Unique Copper And Silver Nitrate Ionic Equation
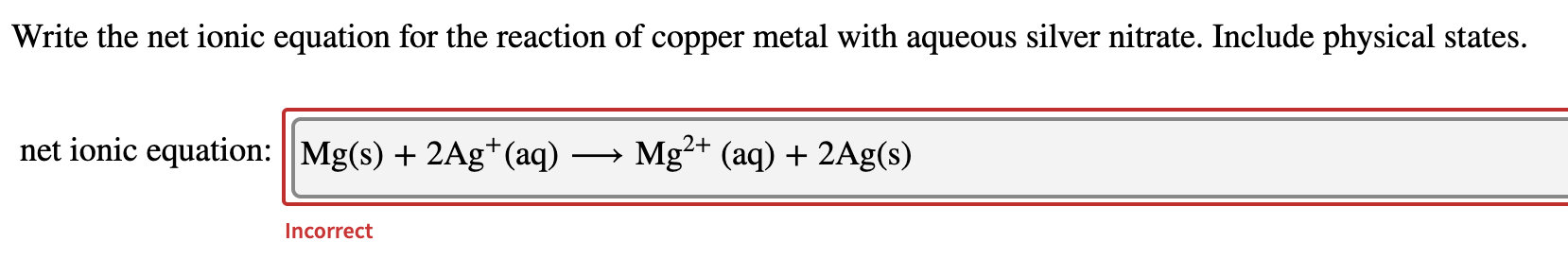
Cu s 2AgNO 3 aq Cu NO 3 2 aq 2Ag s.
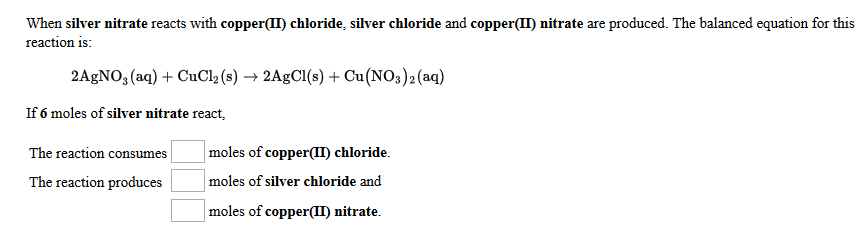
Copper and silver nitrate ionic equation. The balanced equation for this reaction is. When silver nitrate reacts with copper II chloride silver chloride and copper II nitrate are produced. Fill the 250 mL beaker with silver nitrate AgNO 3 solution.
Cu s 2AgNO3aq 2Ag s Cu NO32aq The ionic equation includes all of the ions in the reactants and products. The example below shows the reaction of solid magnesium metal with aqueous silver nitrate to form aqueous magnesium nitrate and silver metal. The reaction of Copper Cu with Silver Nitrate AgNO3 A g N O 3 is an example of displacement reaction full stop Cu being the more reactive metal displaces the weak metal ie.
The reaction between copper and silver nitrate solution. Total-ionic - 3 Sodium ana nitrate. In order to balance AgNO3 Cu CuNO32 Ag youll need to be sure to count all of Ag N O and Cu Cl atoms on each side of the chemical equation.
The balanced equation for the reaction is. NaCl AgNO_3 rightarrow What is the net ionic equation for sodium chloride and silver nitrate. Chemistry project for MrSnyder with Sarah Collins Elizabeth Johnson and my self Brennan Drane.
AgNO_3aqKClaq-AgClsKNO_3aq During this reaction a precipitate will form which is the silver chloride AgCl. Transcribed Image Textfrom this Question. This type of reaction is called a precipitation reaction and the solid produced in the reaction is known as the precipitateYou can predict whether a precipitate will form using a list of solubility rules such as those found in the table below.
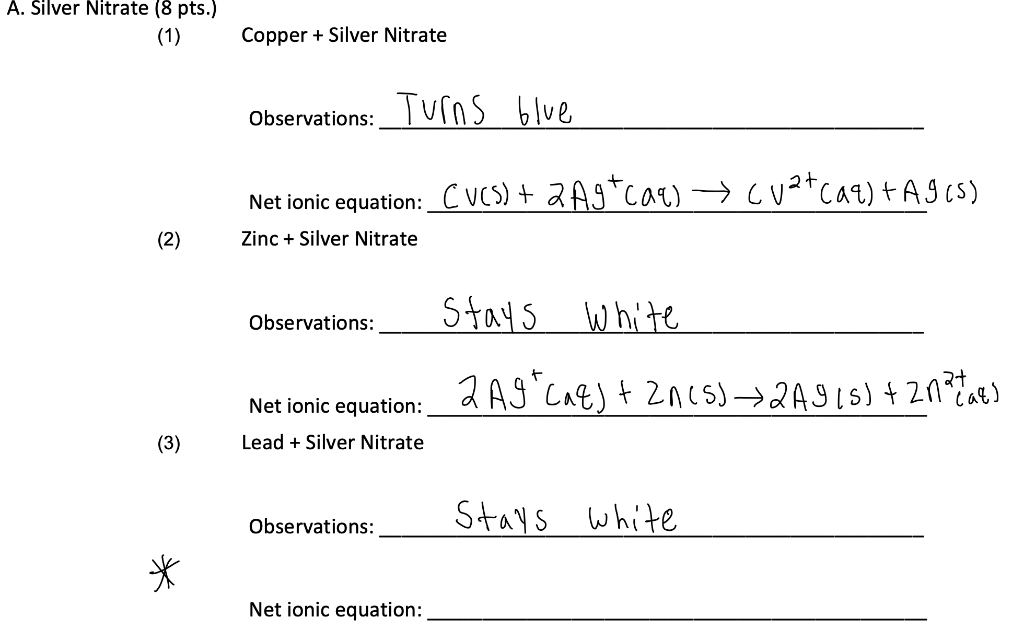
5 arrvmniurn Molecular equation Ba 9 9 Total-ionic Net-Onic equation. Place the coiled copper wire in the solution and observe. Notice the nitrate ions N O 3 they start as aqueous ions and end up exactly the same.