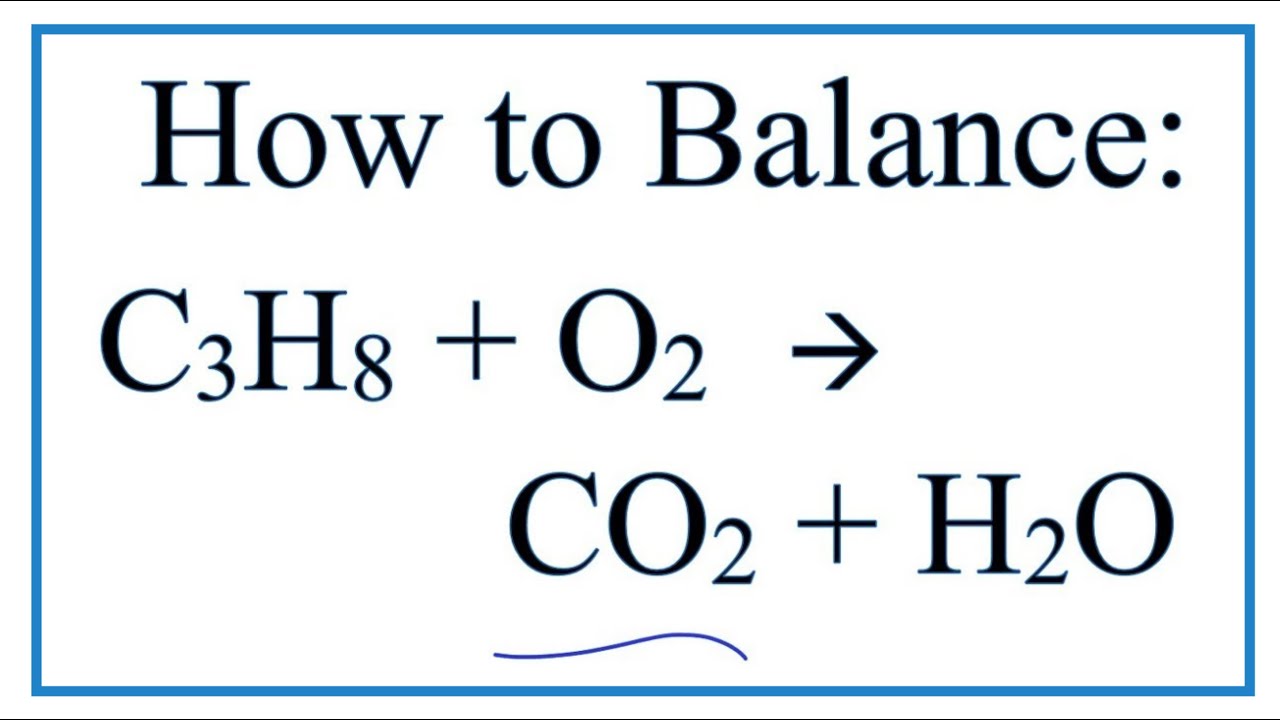
Ideal Balanced Equation Of Propane
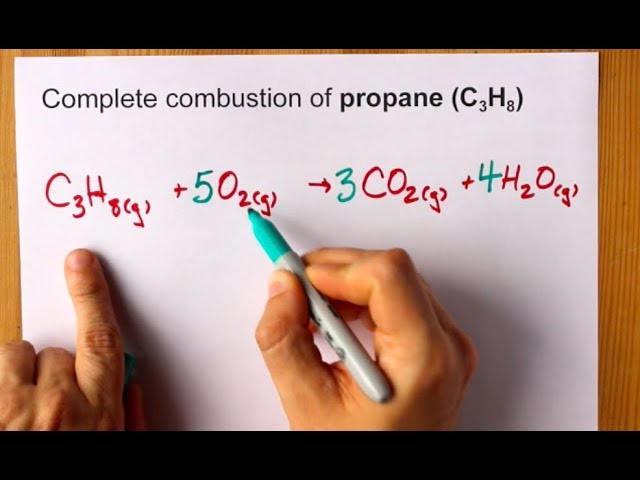
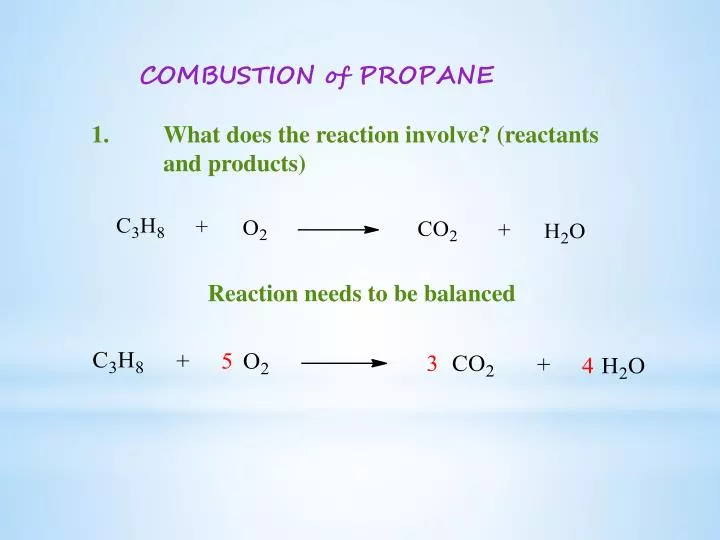
C subscript 3 H subscript 8 space g space plus space 5 space O subscript 2 space g space rightwards arrow 3 space C O subscript 2 space g space plus space 4 space H subscript 2 O space g How many liters of carbon dioxide are produced at STP when 44 g of C3H8 completely reacts with oxygen.
Balanced equation of propane. The limiting reagent row will be highlighted in pink. By signing up youll get thousands of step-by-step solutions to your. This Henrys Law constant indicates that propane is expected to volatilize rapidly from water surfaces 3.
Propane gas reacts with oxygen according to this balanced equation. This means we need 8 hydrogen making the formula C3H 8. What type of reaction is C3H8 O2 CO2 H2O.
DESCRIPTION OF PHYSICAL AND CHEMICAL PROPERTIES OF REACTANTS. For hydrocarbons that end in the suffix -ane the formula is CnH 2n2. VISUAL REPRESENTATION OF BALANCED CHEMICAL EQUATION.
A hydrocarbon is a molecule composed of carbon and hydrogen in this case propane. Fuel O2 -CO2 H2O The coefficients of the balanced equation will change depending on the fuel. The combustion of butane in oxygen produces carbon dioxide and water.
Examples of complete chemical equations to balance. Can be compressed to a. C 3 H 8 5 O 2 3 CO 2 4 H 2 O heat.
Non-toxic three-carbon alkane gas. Balancing chemical reaction balancing combustion of propanec3h8o2--co2h2o. The Henrys Law constant for propane is estimated as 707X10-1 atm-cu mmole SRC derived from its vapor pressure 7150 mm Hg 1 and water solubility 624 mgL 2.