Simple Burning Glucose Reaction
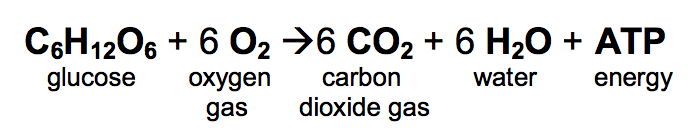
The main difference between respiration and burning is that respiration is the breakdown of glucose to release energy whereas burning is a chemical reaction between a fuel and an oxidant.
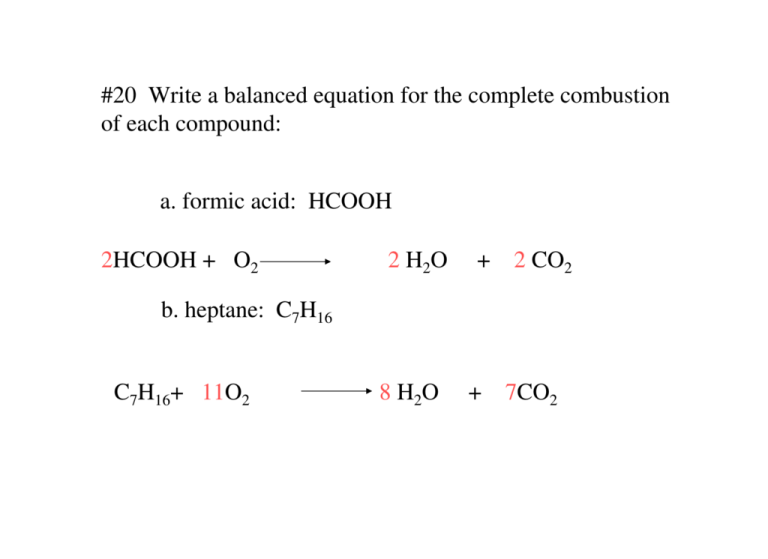
Burning glucose reaction. C6 H12 O6 s6O2 g6CO2 g6H2 Og READ. The equation for the combustion of glucose is. That is they lose electron and go to a higher oxidation state.
Furthermore respiration is a biochemical process occurring inside the cell while burning is a chemical process that is non-cellular. Glucose reacts with molecular oxygen to produce carbon dioxide and water. First lets consider the combustion of sugar or respiration.
During intense exercise your body cannot provide enough oxygen to allow the complete combustion of glucose to carbon dioxide. Ever wondered how much of the air we breath is composed of Oxygen. C 6 H 12 O.
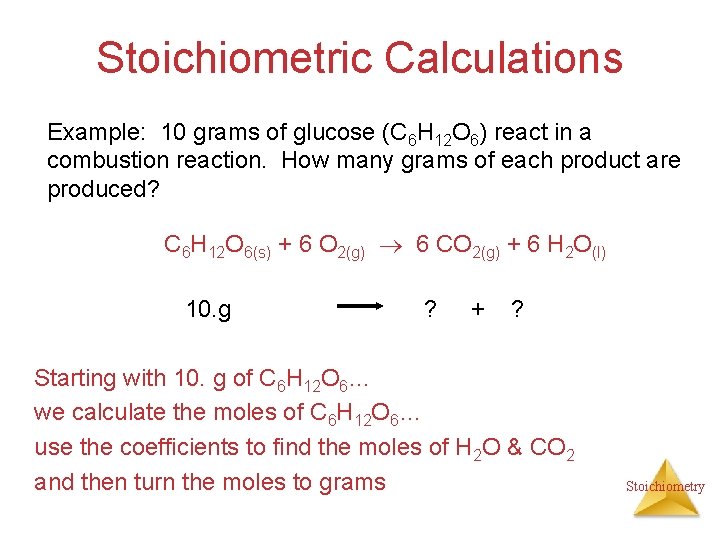
The carbon atoms in glucose are oxidized. From the calculation we know that when one mole or 180 grams of glucose burns it releases 2560 kJ of energy the heat of reaction is -2560 kJmole. How many grams of H2O will be produced when 8064g of glucose is burned.
The equation for this reaction is as follows. The reaction occurs when the heated potassium chlorate decomposes to form potassium chloride and oxygen. It can help but it likely wont eliminate the burning sensation entirely.
Calculating Heat of Combustion. The area before injecting. Chemistry Chemical Reactions Chemical Reactions and Equations 1 Answer.