Beautiful Soda Acid Fire Extinguisher Reaction

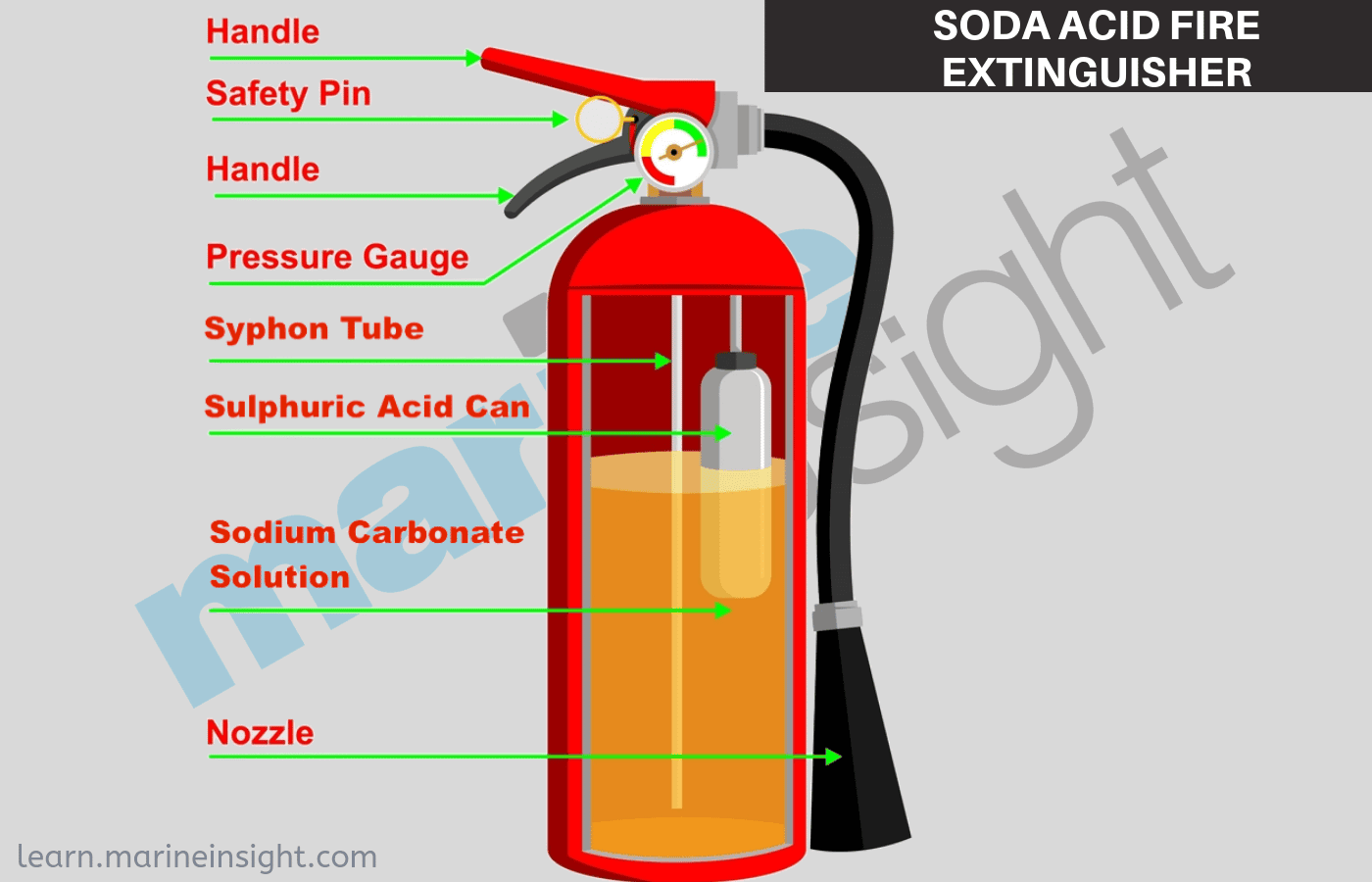
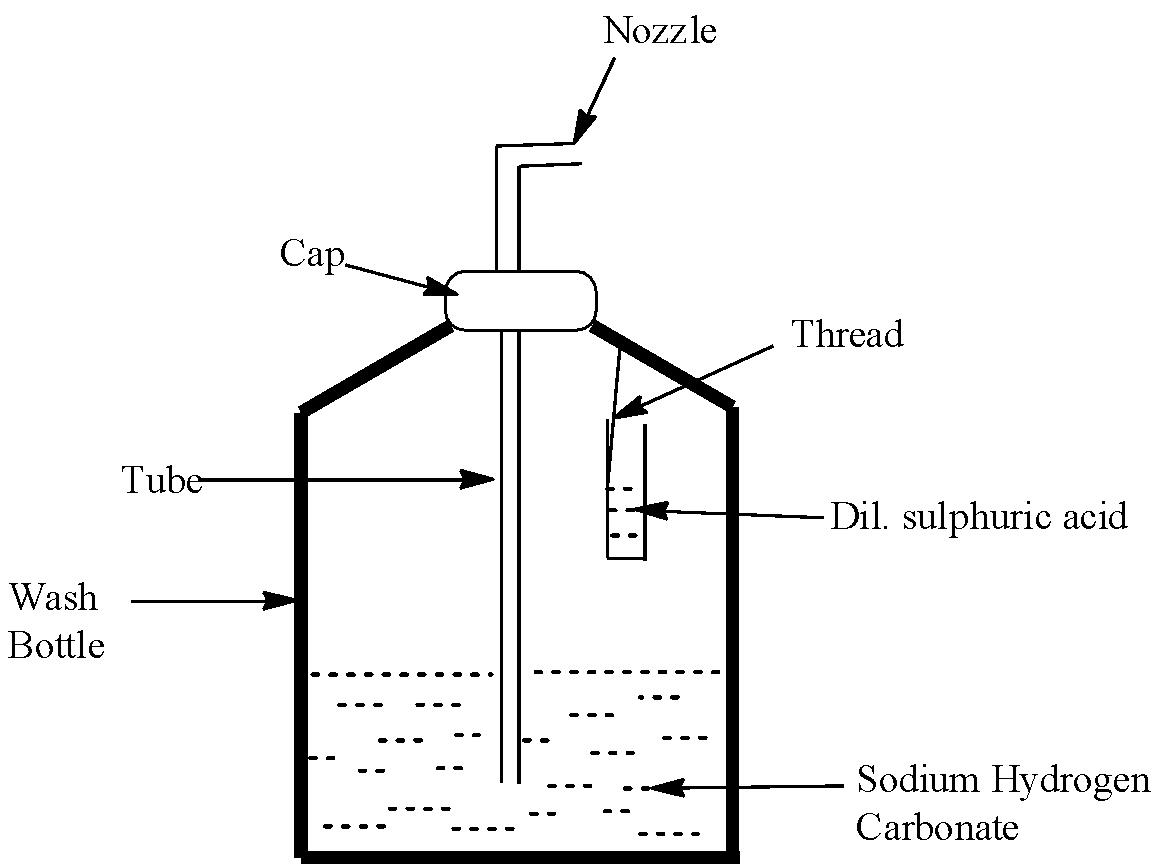
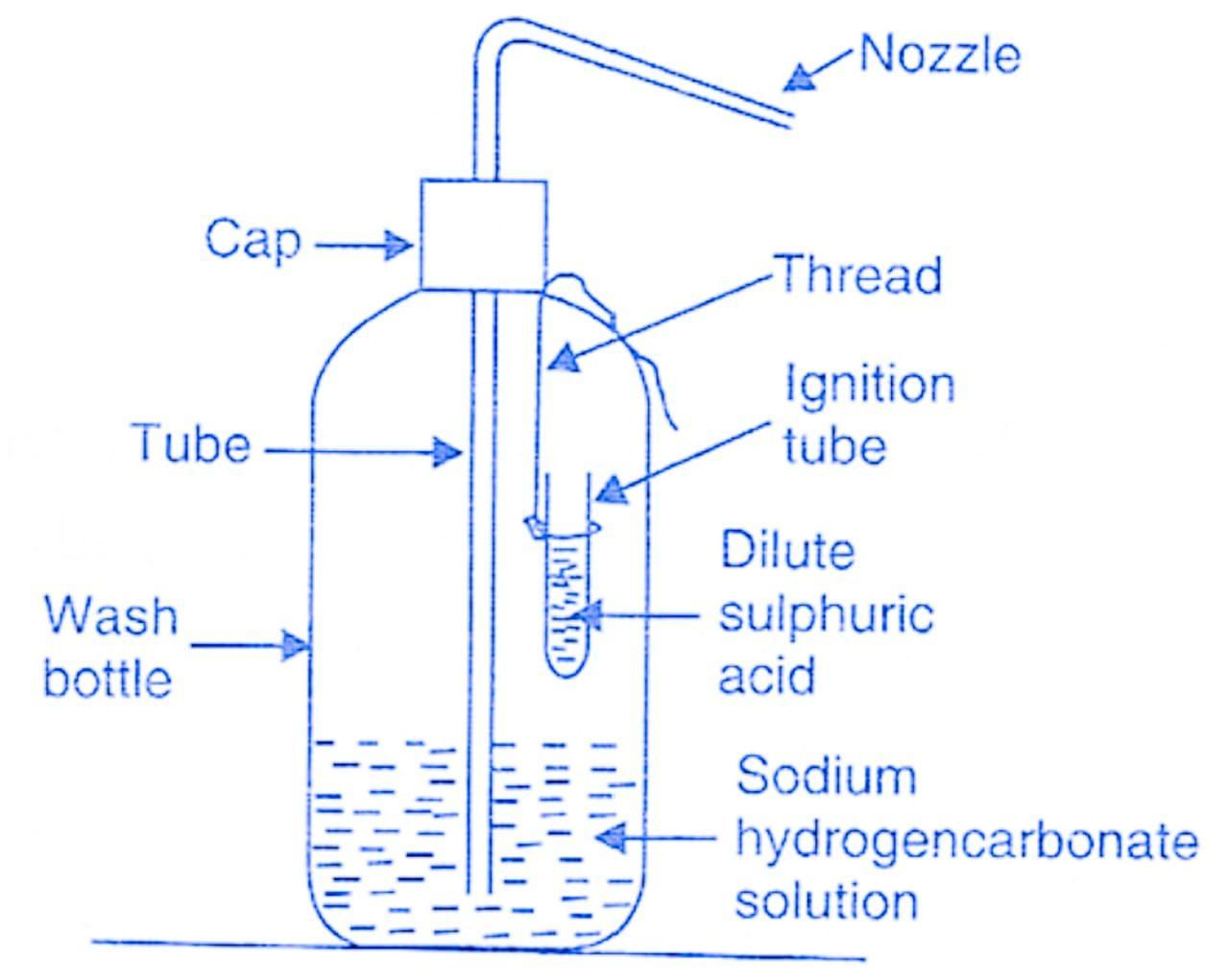
Soda-acid fire extinguisher contains a solution of sodium hydrogen carbonate and sulfuric acid.
Soda acid fire extinguisher reaction. The sulphuric acid reacts with sodium bicarbonate to form sodium. A vial of concentrated sulfuric acid was suspended in the cylinder. There are 4 types of fire extinguisher - soda acid type foam type liquid carbon dioxide type Solid sodium hydrogen.
A soda-acid fire extinguisher system reacts to the sensing of a fire in a building and starts a chemical reaction inside a sealed container. 2NaHCO 3 H 2 SO 4 -------. 2 N a H C O 3 H 2 S O 4 N a 2 S O 4 2 H 2 O 2 C O 2 This increases the percentage of carbon dioxide in air.
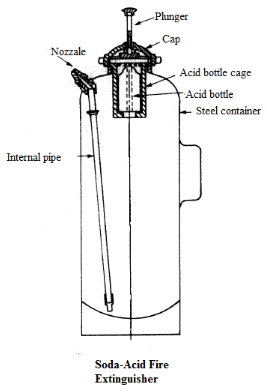
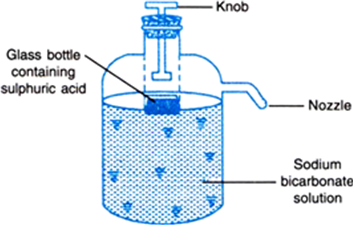
The acid reacted with baking soda dissolved in water when the extinguisher was turned upside down making carbon dioxide gas that propelled the water out of a hose. A knob is fixed just above this bottle. The resulting chemical reaction produces carbon dioxide gas which pressurises the space above the liquid forcing it out through the internal pipe to the nozzle.
When you wanted to use the fire extinguisher you would break the vial via a lever or pick the extinguisher up and slam it on the floor. The chemical reaction produces carbon dioxide and water which is piped to the fire location and is sprayed on the fire by a nozzle. A soda-acid fire extinguisher system reacts to the sensing of a fire in a building and starts a chemical reaction inside a sealed container.
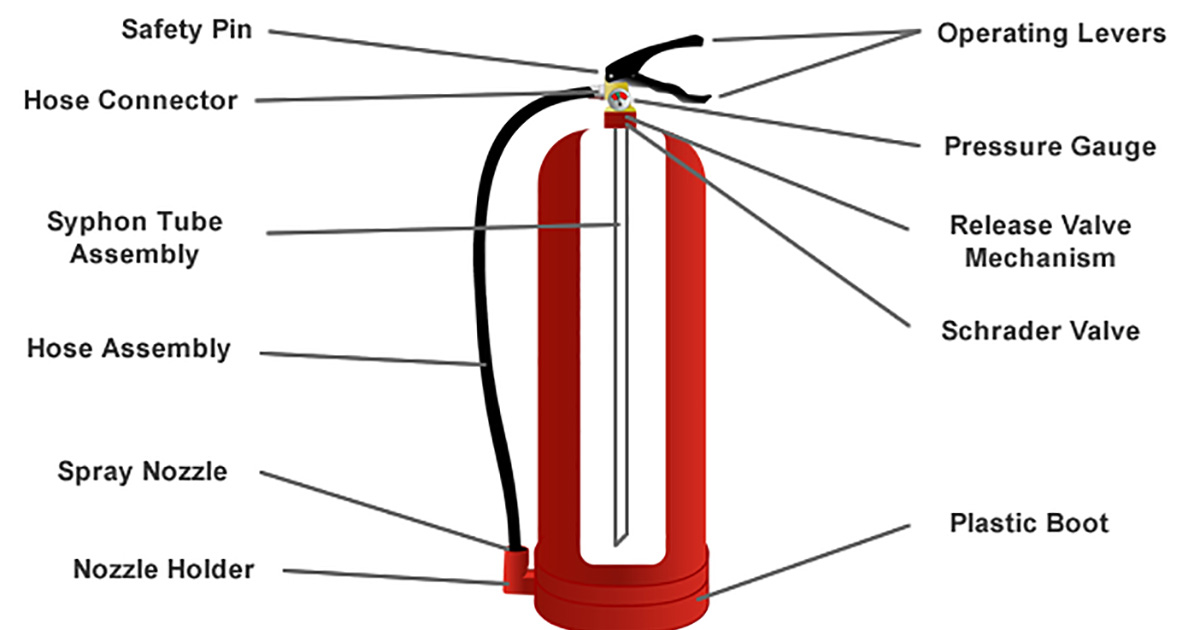
Soda-acid fire extinguishers consist of sodium bicarbonate and sulphuric acid. A fire extinguisher is an active fire protection device used to extinguish or control small fires often in emergency situations. The chemical reaction that takes place in the soda-acid fire extinguisher is as follows.
Subsequent patents have been filed for automatic soda acid fire extinguishers that sense the presence of a fire and start a chemical reaction in the cylinder that produces and propels carbon dioxide CO2 to. When knob of the fire extinguisher is pressed then sulphuric acid mixes with sodium bicarbonate solution and produces a lot of CO2 gas which forms a blanket over the fire and cuts it off from the supply of the air to the burning substance and the fire stops. Although no longer used soda acid extinguishers were used on Class A ordinary combustibles fires such as wood paper and cloth.