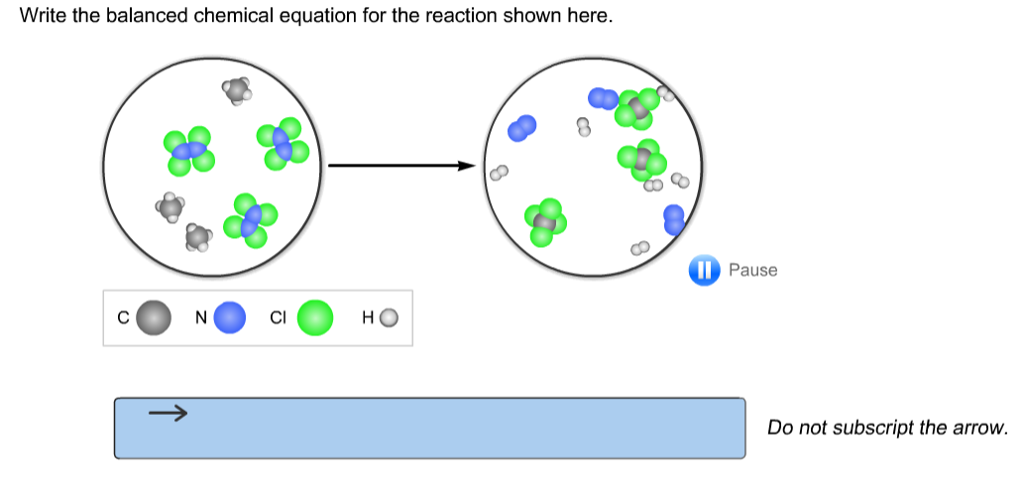
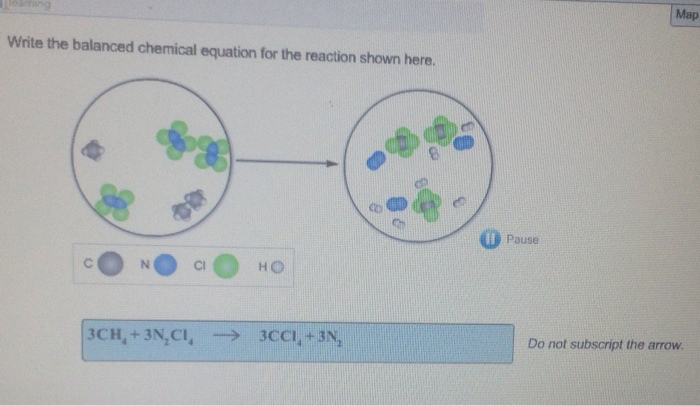
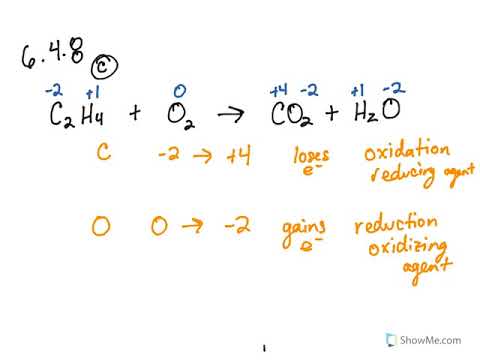
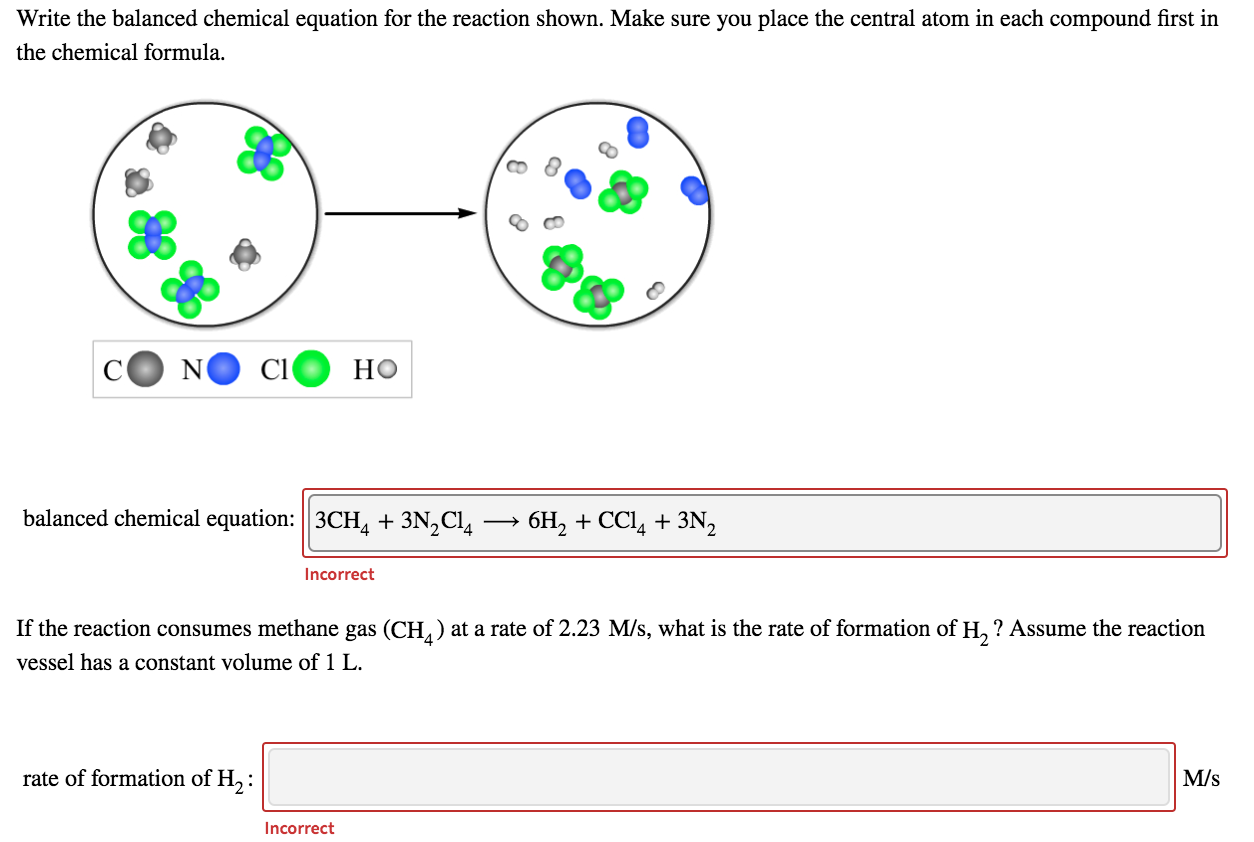
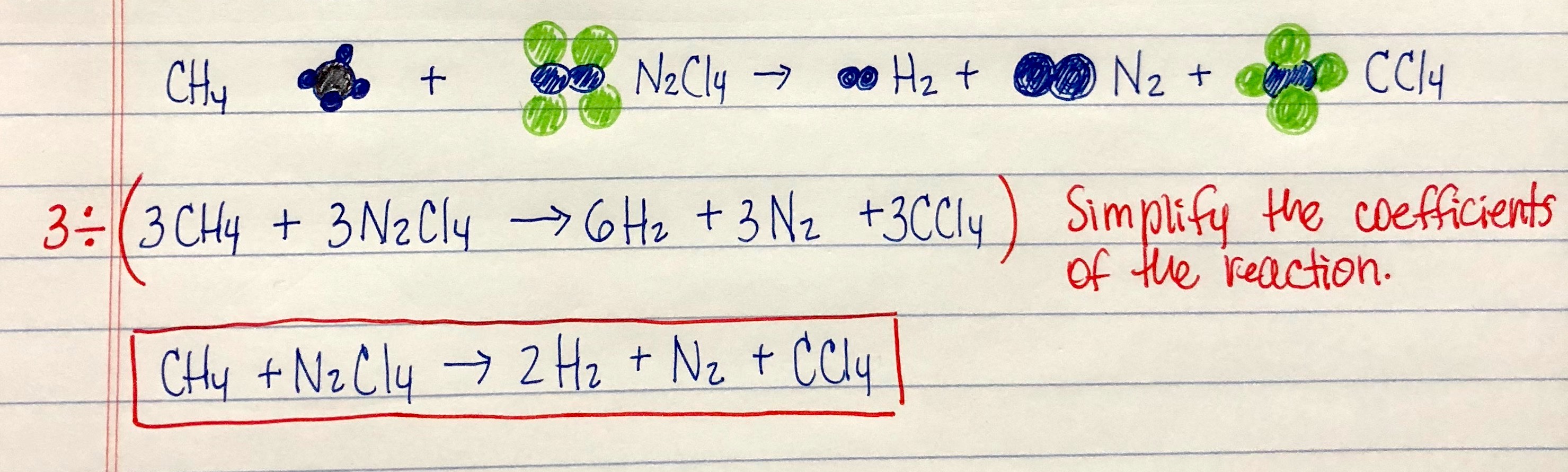Brilliant Write The Balanced Chemical Equation For The Reaction Shown Here
Plaster of Paris when reacts with water liberating heat through crystallization.
Write the balanced chemical equation for the reaction shown here. When we balance reaction equations we may ONLY add coefficients to the chemical formulae that are already in the equation. Write and balance chemical equations in molecular total ionic and net ionic formats. Writing balanced chemical equations is essential for chemistry classHere are examples of balanced equations you can review or use for homework.
41Writing and Balancing Chemical Equations By the end of this section you will be able to. Exam 2 Unit 4 Write the balanced chemical equation for the reaction shown here. Identify the number of each atom in the reactants and products for this balanced reaction.
Here we will consider titrations that involve acid-base reactions. And likewise when you make an electronic transaction I would imagi. Derive chemical equations from narrative descriptions of chemical reactions.
Make sure you place the central atom in each compound first in the chemical formula. A titration can be performed with almost any chemical reaction for which the balanced chemical equation is known. To balance a chemical equation enter an equation of a chemical reaction and press the Balance button.
The word equations for a few of these reactions have been provided though most likely youll be asked to provide only the standard chemical equations. A balanced reaction equation must have the same number of atoms of each element on both sides of the equation. C1 HO balanced chemical equation.
The unbalanced chemical equation that describes this neutralization reaction looks like this CH_ 3COOH_ aq NaOH_ aq - CH_ 3COONa_ aq H_ 2O_ l Now you could check to see if this chemical equation is balanced by counting the number of atoms of each element present on both sides of the equation or you could. Use uppercase for the first character in the element and lowercase for the second character. Automobile airbags inflate due to the formation of nitrogen gas from the following chemical reaction.