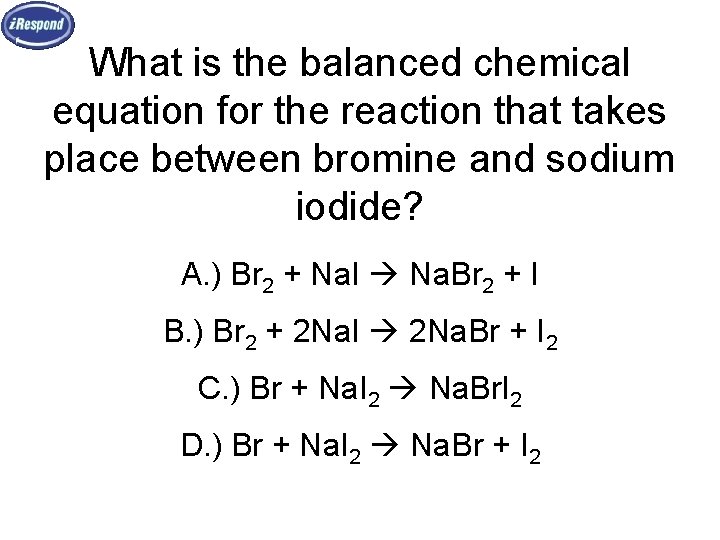Looking Good Sodium And Bromine Balanced Equation

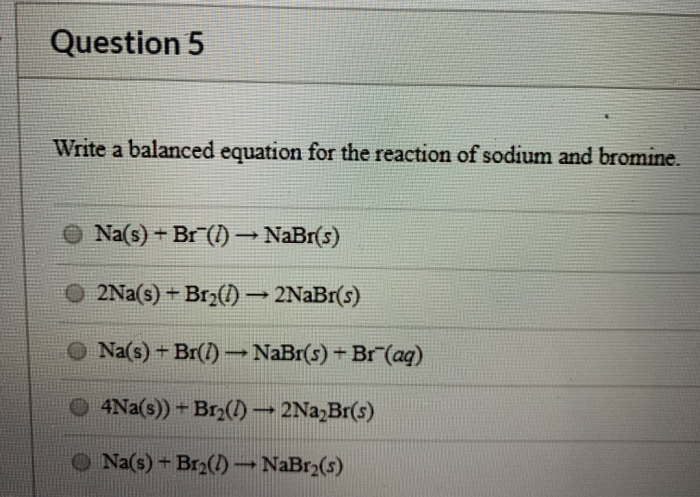
What is the balanced chemical equation for the synthesis of sodium bromide from sodium and bromine.
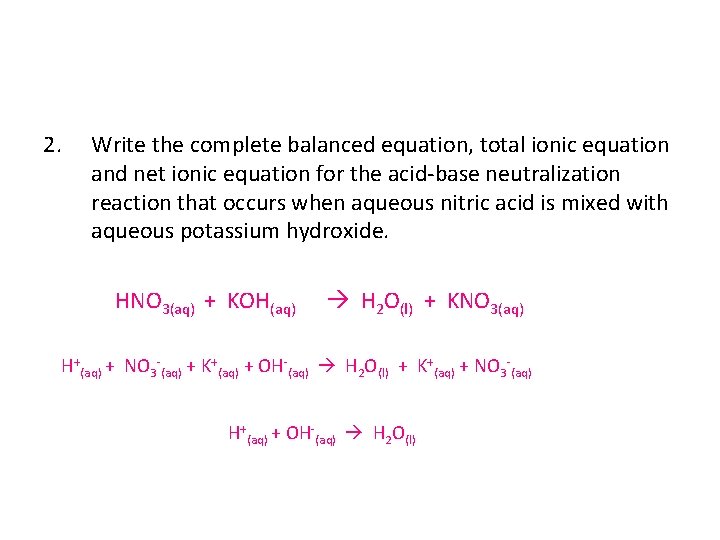
Sodium and bromine balanced equation. So Bromine bromine is in group 7 meaning it needs one electron to become stable need 1 electron to be stable whilst Sodium sodium is in group1 meaning it has one lone electron need to lose one electron. Write a balanced equation for the following reaction. Chemistry Chemical Reactions Balancing Chemical Equations.
Solid sodium bromide decomposes into bromine gas and solid sodium. Bromine - saturated aqueous solution. Kathryn Wright answered In short 2NaI Br2------ 2NaBr I2 The balanced equation of bromine plus sodium iodide yields sodium bromide plus iodine is2NaI Br2 ------ 2NaBr I2Two moles of sodium iodide react with one mole of bromine to form two moles of sodium bromide and one mole of iodine.
Na Br2 NaBr Chemical Equation Balancer. Sodium hydroxide hydrochloric acid sodium chloride water NaOH HClaq NaCl H2O methathesis 5. A potassium hydroxide sodium sulfide -- potassium sulfide sodium hydroxide b carbon disulfide oxygen -- carbon dioxide sulfur dioxide.
The formula of sodium bromide is NaBr The formula of sodium is Na. Use whole number coefficients and subscripts as appropriate. Na 2 S 2 O 3 4Br 2 5H 2 O Na 2 SO 4 H 2 SO 4 8HBr Check the balance Sodium thiosulfate react with bromine and water to produce sodium sulfate sulfuric acid and hydrogen bromide.
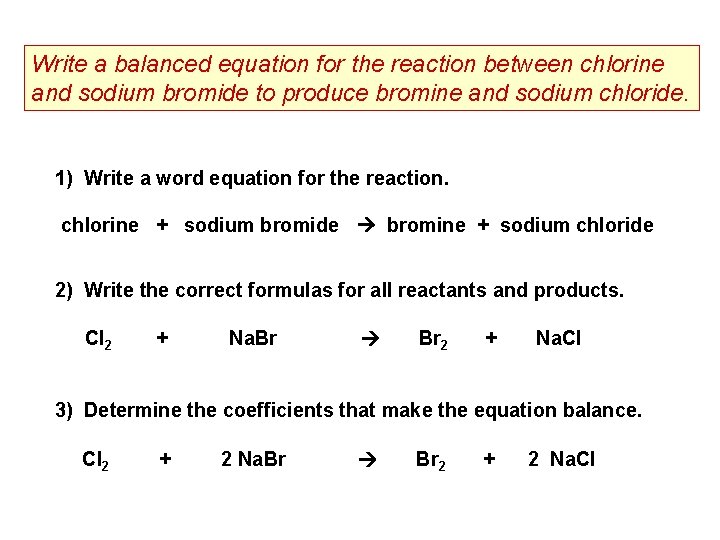
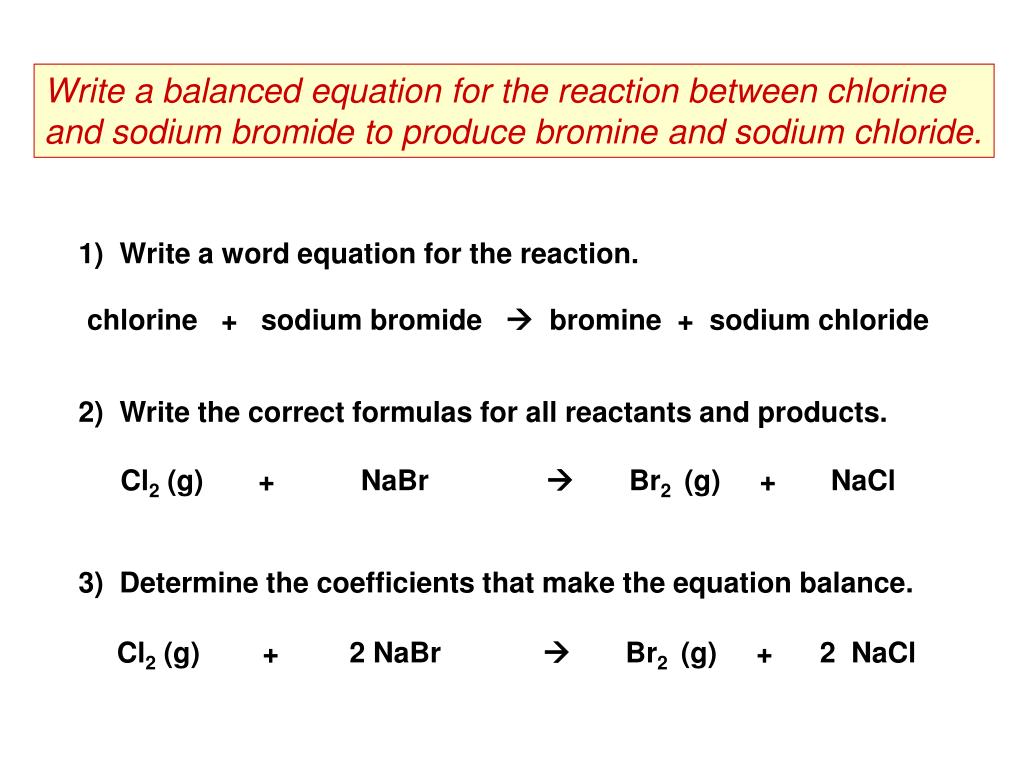
Because chlorine is more reactive than bromine it displaces bromine from sodium bromide. To do soat firstwe have to know the formula of sodium bromidesodium and chlorine. 2 moles of water and.
Magnesium bromide chlorine magnesium chloride bromine MgBr2 Cl2 MgCl2 Br2 replacement. When brown bromine solution bromine water is added to colourless sodium sulfite solution the bromine rapidly loses its colour and sulfite ions change into colourless sulfate ions. 1 Answer anor277 Aug 1 2017 Nas 12Br_2l rarr NaBrs.