Peerless Burning Of Gasoline Chemical Equation

Equation to simulate the radiation from the flame P-1 radiation model.
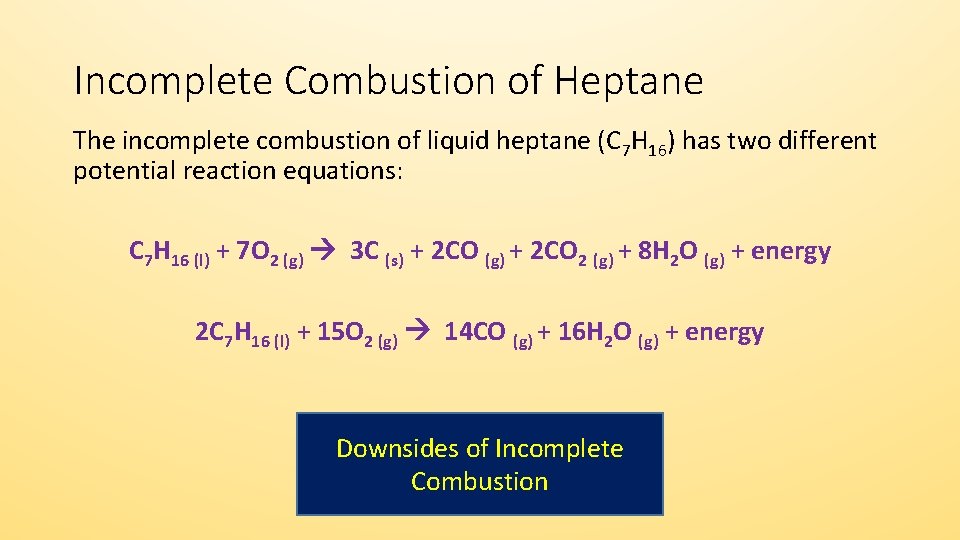
Burning of gasoline chemical equation. The combustion of methane is represented by the equation. Burning gasoline - When we burn gasoline we are combusting it or combining it with oxygen. In complete combustion of acetylene gas C2H2 C2 H 2 4 302 2 C0 HO 07 1 02 -Y 2 2C HO an a on complete combustion it gives coz while in by on incomplete.
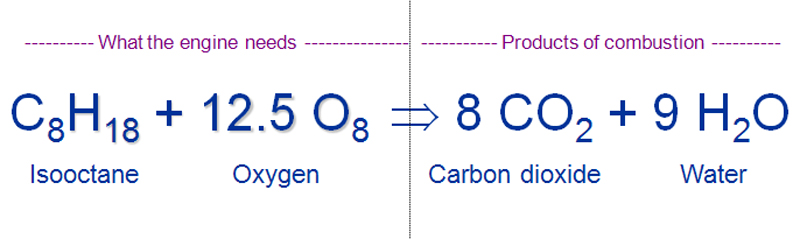
For example hydrogen gas H 2 can react burn with oxygen gas O 2 to form water H 2 0. Gasoline is usually approximated as being made up of only octane whose chemical formula is C8H18. C 2 H 5 OH Chemical Formula Combustion Crude Oil Ethanol Ethyl Alcohol Fuel Gasoline What is Ethanol.
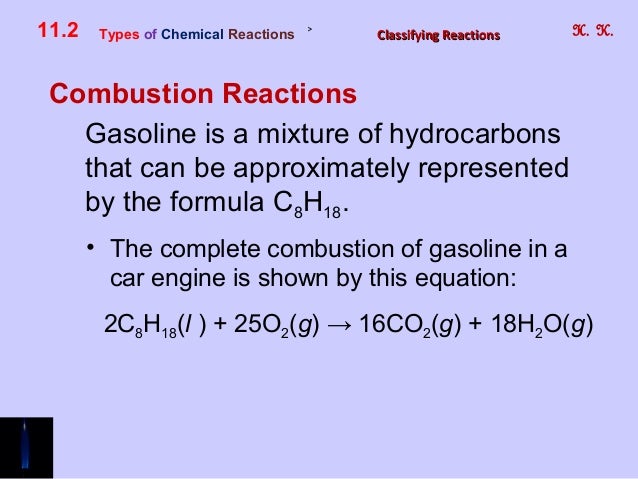
Write the balanced chemical equation for burning gasoline assume octane reacting with oxygen. The combustion of methane gas heats a pot on a stove. By Stoichiometry of the reaction.
9The burning of gasoline to power internal combustion engines produces water vapor and carbondioxide gas that is thought to contribute to the Greenhouse Effect in the earths atmosphere. For steady turbulent non premixed combustion 3 the time averaged gas phase equations are summarized in Table 1. Barrans Jr Newton Ask-a-scientist.
CH4 2O2 CO2 2H2O. This reaction is known as combustion reaction. Like most hydrocarbons octane reacts with oxygen gas to produce carbon dioxide and water.
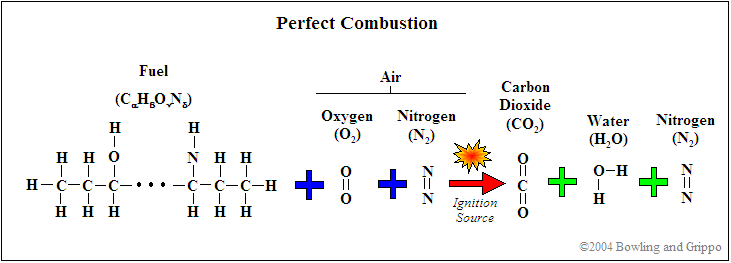
This is typical of combustion reactions involving hydrocarbons such as octane and propane. What is the balanced chemical equation for burning methane gas. Hydrocarbons HCs are any molecules that just contain hydrogen and carbon both of which are fuel molecules that can be burned to form water H2O or carbon dioxide CO2.